JEE Class main Answered
?plz explain option 2 & 3 thanks!??
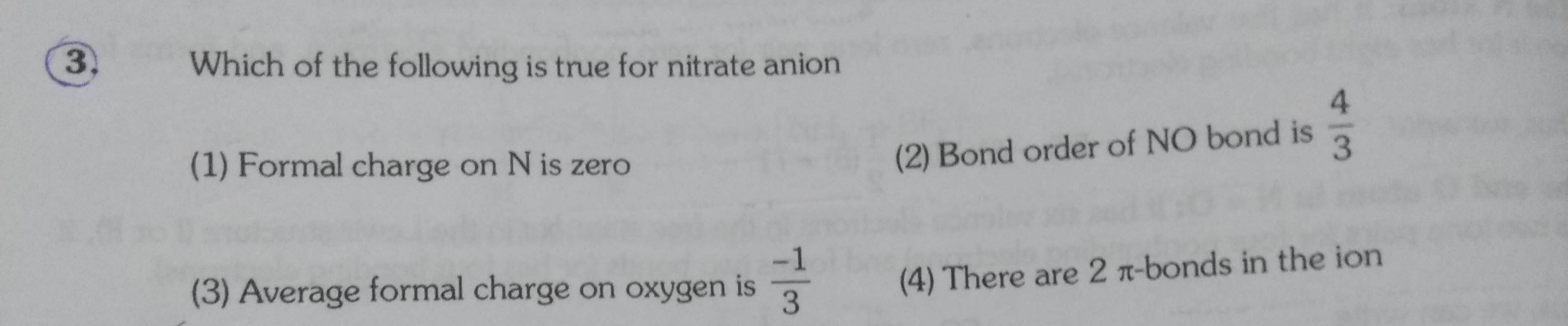
Asked by vidyavikram10 | 21 Aug, 2020, 21:40: PM
(2) The resonating structures of nitrate ion NO3- are: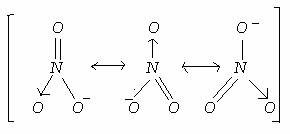
Bond order of NO bond is = 4/3 = 1.33 (Total number of bonds/Resonating structures)
(3) Formal charge=+ or - [Number of valence electron on atom -(non-bonded electrons+number of bonding electrons/2)]
So, Here three O will have separate formal charges, because three of them are having different number of bonds and non-bonded electrons.
Formal charges of O
1st(with double bond), 6-[4+2/2]=-1 (-because O is non-metal gains the electron))
2nd(with -1 Charge), 7-[6+1/2]=-1/2
3rd, 6-[5+1/2]=-1/2
So, average formal charge of O would be= -2/3
Average formal charge would be
Answered by Ravi | 22 Aug, 2020, 19:21: PM
JEE main - Chemistry
Asked by gattimadhavi434 | 25 Dec, 2023, 10:15: AM
JEE main - Chemistry
Asked by visalvinod85 | 23 Jun, 2022, 08:41: AM
JEE main - Chemistry
Asked by deba.biswas561 | 19 Jun, 2022, 09:00: AM
JEE main - Chemistry
Asked by rakeebalikcl | 13 Jun, 2022, 05:47: AM
JEE main - Chemistry
Asked by pachchigarkeyur | 25 Mar, 2022, 18:09: PM
JEE main - Chemistry
Asked by pachchigarkeyur | 22 Mar, 2022, 12:37: PM
JEE main - Chemistry
Asked by pachchigarkeyur | 22 Mar, 2022, 12:35: PM
JEE main - Chemistry
Asked by ojili005 | 31 May, 2021, 19:20: PM


