CBSE Class 10 Answered
10g of hydrogen is brunt in the presence of excess oxygen . The mass of water formed is
Asked by gharatpooja70 | 22 Jun, 2020, 08:22: PM
Burning of hydrogen is written as-
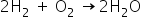
10 g of H2 means 10/2=5 moles of H2.
According to stoichiometry,

Answered by Ravi | 22 Jun, 2020, 10:14: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by shoaibhakak41 | 18 Jan, 2023, 02:17: PM
CBSE 10 - Chemistry
Asked by nehashekh291 | 03 Jan, 2023, 03:11: PM
CBSE 10 - Chemistry
Asked by ansheera3236 | 11 Jul, 2022, 05:05: PM
CBSE 10 - Chemistry
Asked by pachchigarkeyur | 08 Mar, 2022, 12:05: PM
CBSE 10 - Chemistry
Asked by radhikaraut258 | 25 Feb, 2022, 09:07: AM
CBSE 10 - Chemistry
Asked by siwach.mukesh78 | 03 Nov, 2021, 08:48: AM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 23 Sep, 2021, 01:57: PM
CBSE 10 - Chemistry
Asked by abhishek707356 | 10 Sep, 2021, 10:29: AM