ICSE Class 9 Answered
100 mL of 0.2 N HCl is added to 100 mL of 0.18 N NaOH and the whole volume is made 2 liters.The pH of resulting solution is
Asked by dev.sultanpur123 | 03 May, 2015, 10:30: PM
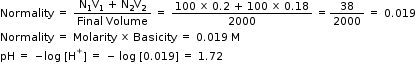
Answered by Arvind Diwale | 04 May, 2015, 10:01: AM
Concept Videos
ICSE 9 - Chemistry
Asked by gangolykavita890 | 08 Mar, 2024, 08:32: PM
ICSE 9 - Chemistry
Asked by zairafathma933 | 22 Dec, 2023, 11:57: PM
ICSE 9 - Chemistry
Asked by mangalgourihebballi | 27 Oct, 2023, 12:32: PM
ICSE 9 - Chemistry
Asked by suhanipiplani13 | 07 Jul, 2023, 10:45: PM
ICSE 9 - Chemistry
Asked by ootysmh1 | 21 Dec, 2022, 09:03: PM
ICSE 9 - Chemistry
Asked by ns175992 | 17 Jul, 2022, 06:57: AM
ICSE 9 - Chemistry
Asked by abbadgaming | 03 Jul, 2022, 11:06: PM
ICSE 9 - Chemistry
Asked by ayantanchoudhuri | 26 Jun, 2022, 06:47: PM
ICSE 9 - Chemistry
Asked by purovidutta | 18 Jun, 2022, 09:27: AM
ICSE 9 - Chemistry
Asked by mantashatakey786 | 23 Oct, 2021, 09:53: PM






