NEET Class neet Answered
Which has maximum number of molecules?
1) 7 gmN2 2) 2 gmH2 3) 16 gmNO2 4) 16 gmO2
Asked by Amrithahr4 | 20 Apr, 2020, 02:51: PM
We know,
No. of moles =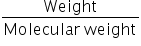
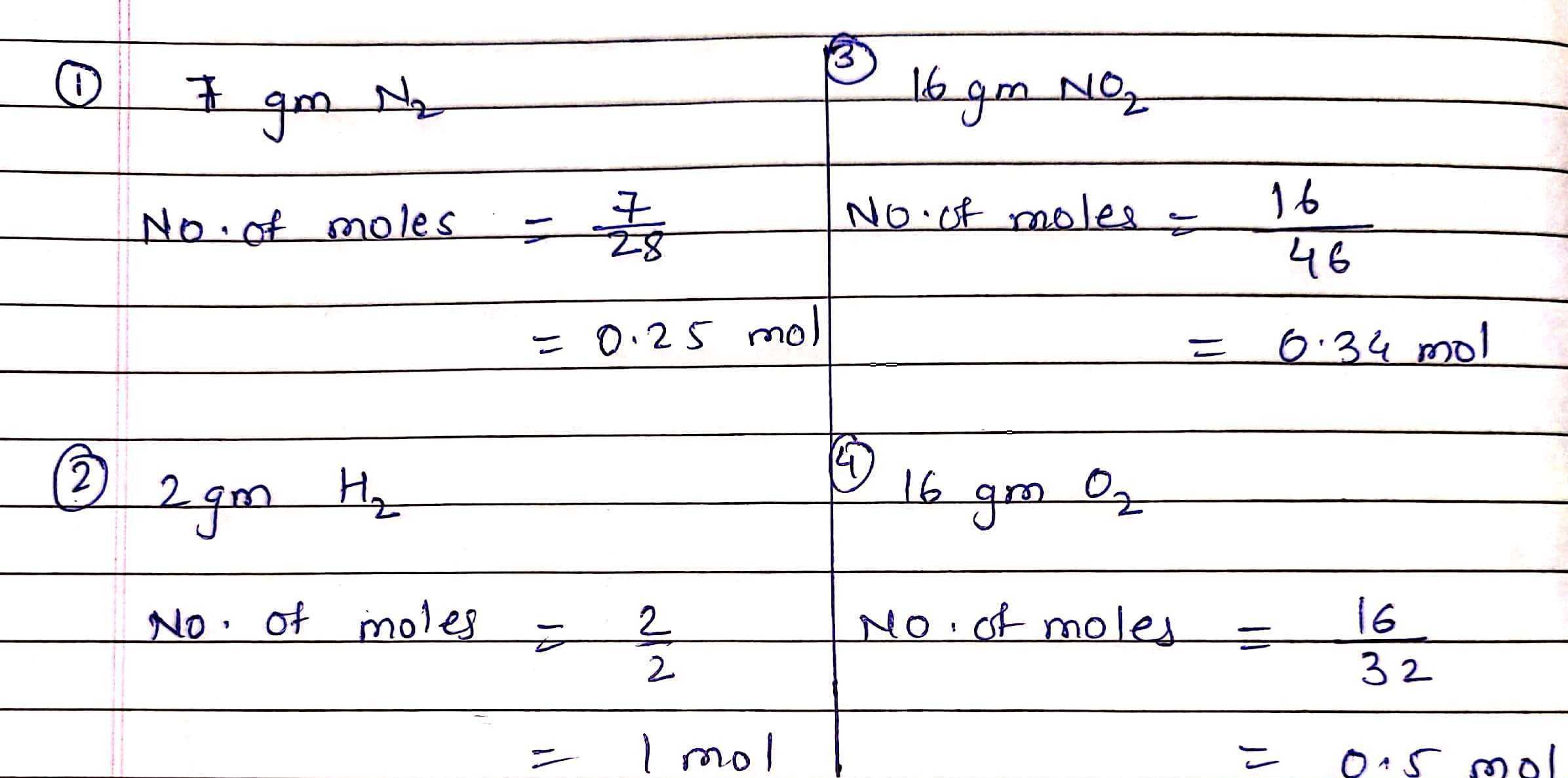
As the number of moles is directly proportional to number of molecules.
So 2 gm of H2 will contain maximum number of molecules.
Answered by Varsha | 21 Apr, 2020, 04:01: PM
Application Videos
Concept Videos
NEET neet - Chemistry
Asked by biswassayan8464 | 21 Apr, 2024, 11:30: AM
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by raomayankup83 | 15 Apr, 2024, 07:46: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
Asked by vasantagomasi23 | 05 Apr, 2024, 08:35: AM
NEET neet - Chemistry
Asked by ankuruthanuriya | 03 Apr, 2024, 10:56: PM




















