CBSE Class 12-science Answered
Sir, Please give me a detailed description of why the d-orbitals split when the negative field due to ligands surrounds the metal atom/ion? How does it become assymetrical?
Please give detailed explanation of the very same.
Asked by Ishita Puranik | 25 Dec, 2011, 12:51: PM
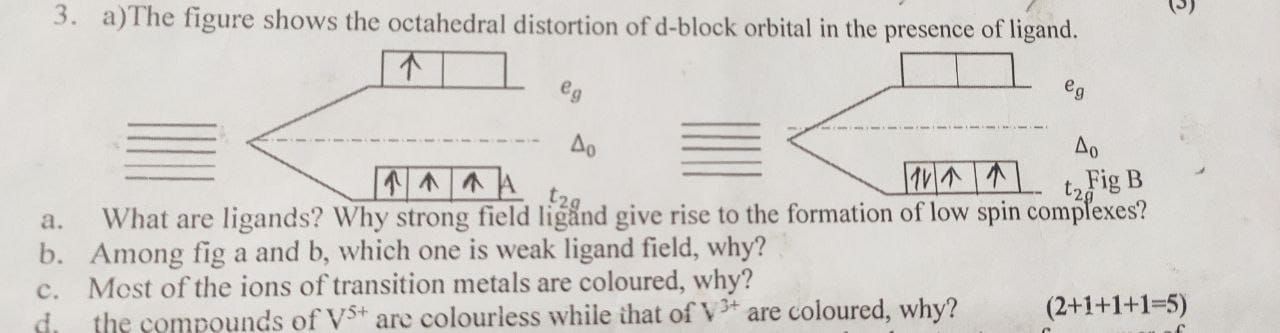
Under the experience of negative fied due to ligands or neutral molecules which have lone pair of electrons,the d-orbitals split into two levels i.e.t2g and eg.
When the complex is formed, the ligands destroy the degeneracy(same energy level) of these orbitals i.e.the orbitals have now different energies.In an isolated gaseous metal ion the five d-orbitals do all have same energy termed as degenerate.However,the energy of orbitals is raised because of repulsion between the field and the electrons on the metal.In most transition metal complexes either six or four ligands surround to metal giving octahedral or tetrahedral structures.
In these two cases the field produced by the ligand is not spherically symmetrcal.Thus,the d-orbitals are not all affected equally by ligand field and thus become asymmetrical.
Answered by | 25 Dec, 2011, 01:15: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by navadeepnavadeep242 | 19 Mar, 2024, 08:56: PM
CBSE 12-science - Chemistry
Asked by chaudharyanu1113 | 01 Feb, 2024, 05:12: PM
CBSE 12-science - Chemistry
Asked by dabhaniamurta | 10 Jan, 2024, 07:26: AM
CBSE 12-science - Chemistry
Asked by arjunsah797 | 13 May, 2022, 06:50: PM
CBSE 12-science - Chemistry
Asked by arjunsah797 | 10 May, 2022, 12:16: PM
CBSE 12-science - Chemistry
Asked by shivubh161 | 24 May, 2021, 03:39: PM
CBSE 12-science - Chemistry
Asked by fishtailfever | 21 Feb, 2021, 02:07: PM
CBSE 12-science - Chemistry
Asked by prathyushagn1 | 09 Dec, 2020, 08:12: AM
CBSE 12-science - Chemistry
Asked by mahesh.h.s2003 | 20 Oct, 2020, 08:47: PM
CBSE 12-science - Chemistry
Asked by ABHILASHA | 31 Aug, 2020, 08:24: PM