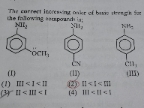
NEET Class neet Answered

The answer would be option 2
Explanation:
Due to electronic repulsion has lowest EA in the group
and Cl has largest EA among halogens
and O– has more ie than S–
you can learn this order for IE
10>9>7>8>6>4>5>3
This is the order for IE where the numbers are corresponding atomic numbers of elements
If the second-period element or any elements is asked from whichever period you add the corresponding +8 or +18 to these numbers to get the IE order for that period
here O– has 9 electrons and S– has 17
both fall in the same position
according to order but IE decreases down the group so O– has more IE than S–
according to hund's rule, electrons occupy different orbitals in nitrogen. 2P orbital of nitrogen is half filled. while in oxygen there are 4 electrons in 2P orbital. electrons in one of the 2P orbital in oxygen is paired which repel each other and so their attraction becomes low. so it is easy to lose this most loosely held electron which require less energy. it is the reason that first ionisation energy of Oxygen lower than that of Nitrogen.
The elstronc arrangement in nitrogen id