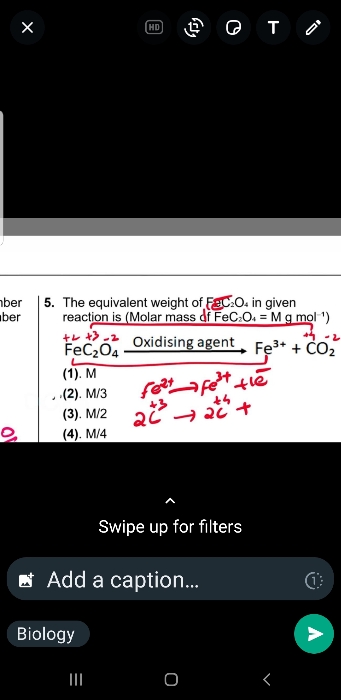
NEET Class neet Answered
please answer this

Asked by Prashant DIGHE | 06 Jan, 2020, 09:56: PM
Option (1) is correct.
Given:
Density = 1.07 g/ml
Molarity = 2.03 M
Molar mass of acetic acid = 60.
We know,
Molarity
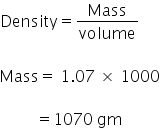
Mass of solution = 1.07 kg
No. of moles = 2.03 mole
As molarity is no. of moles of solute in 1000 ml of solution.
No. of moles 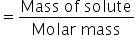
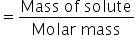
Mass of solute = No. of moles × Molar mass
= 2.03 × 60
Mass of solute (CH3COOH) = 121.8 gm
Mass of solvent = 1070 − 121.8 = 948.2 gm
= 0.9482 kg
Molality 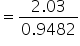
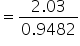
= 2.15 m
Molality ≈ 2.2 m
Answered by Varsha | 07 Jan, 2020, 01:12: PM
NEET neet - Chemistry
Asked by biswassayan8464 | 21 Apr, 2024, 11:30: AM
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
Asked by vaka.aruna1979 | 23 Mar, 2024, 04:18: AM
NEET neet - Chemistry
Asked by fathimahusna23042004 | 03 Mar, 2024, 08:56: AM
NEET neet - Chemistry
Asked by drkeshavkhandagle | 18 Jan, 2024, 08:10: PM
NEET neet - Chemistry
Asked by yogitakumawat | 21 Dec, 2023, 10:31: PM