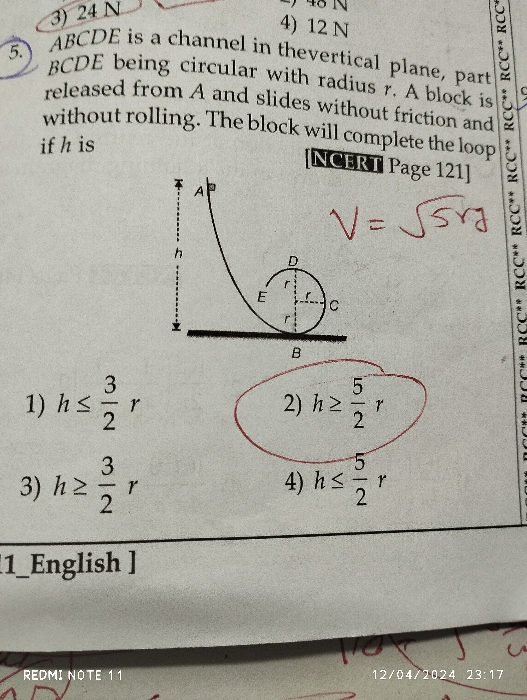NEET Class neet Answered
please answer this
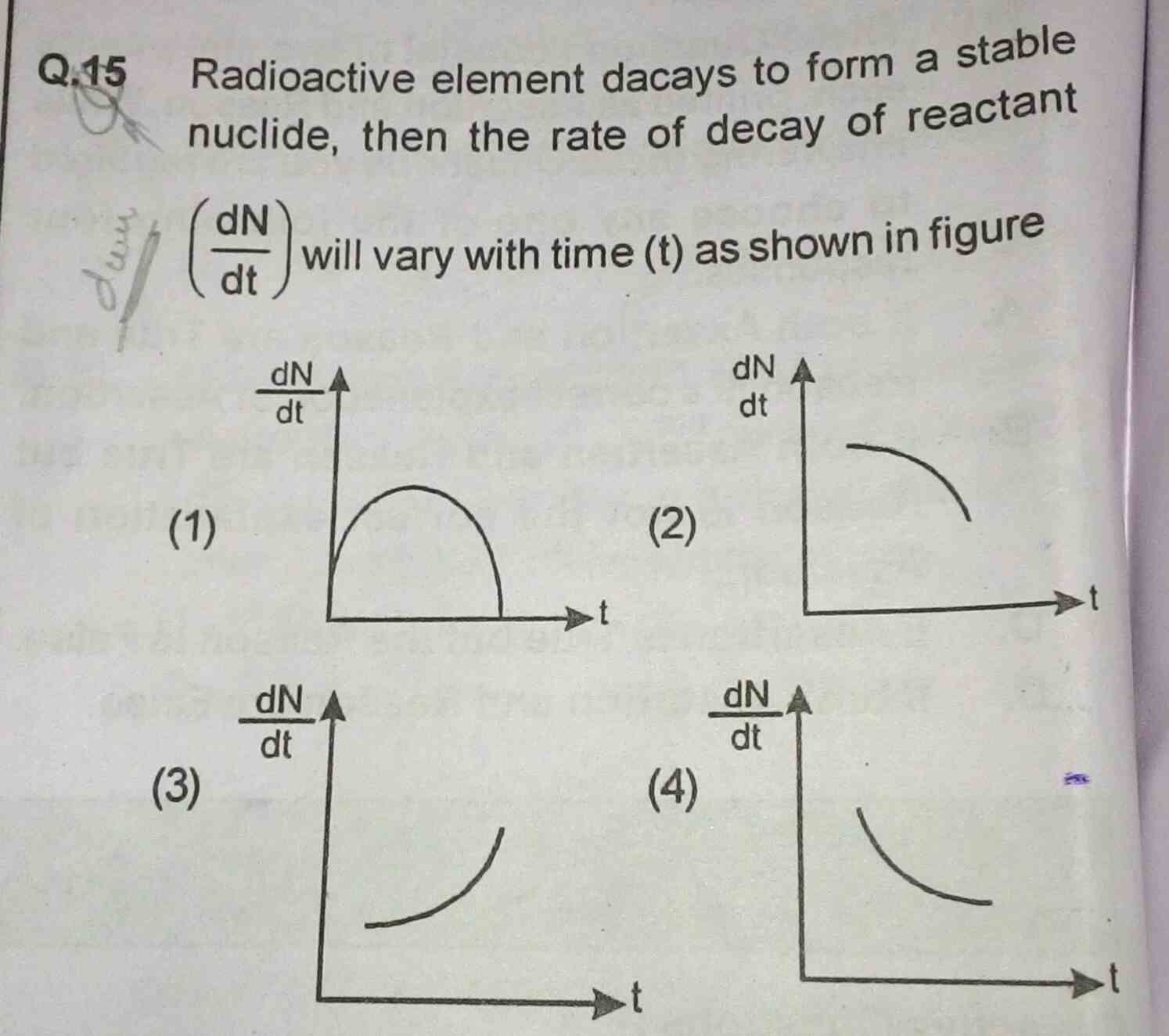
Asked by Prashant DIGHE | 15 Jan, 2020, 10:16: PM
For radioactive decay, rate of disintegration is proportional to number of undecayed atoms present.
dN/dt = -λN ........................... ( 1 )
where λ is decvay constant, -ve sign indicates rate of disinegeration decreases.
we have radioactive decay equation as, N = No e-λt .....................(2)
where No is the number of undecayed atoms present at t=0.
hence from eqn.(1) and (2), we get, | dN/dt | = λ No e-λt
Hence figure (4) shows the correct variation of rate of disintegration as a function of time.
Answered by Thiyagarajan K | 15 Jan, 2020, 10:57: PM
Application Videos
NEET neet - Physics
Asked by ramanjaneyuluoguru | 25 Apr, 2024, 04:18: PM
NEET neet - Physics
Asked by shatakshibhatt9 | 20 Apr, 2024, 07:52: PM
NEET neet - Physics
Asked by praveenpriya000079 | 18 Apr, 2024, 07:24: AM
NEET neet - Physics
Asked by gouranshi84 | 17 Apr, 2024, 05:23: PM
NEET neet - Physics
Asked by sojusvi | 17 Apr, 2024, 01:12: PM