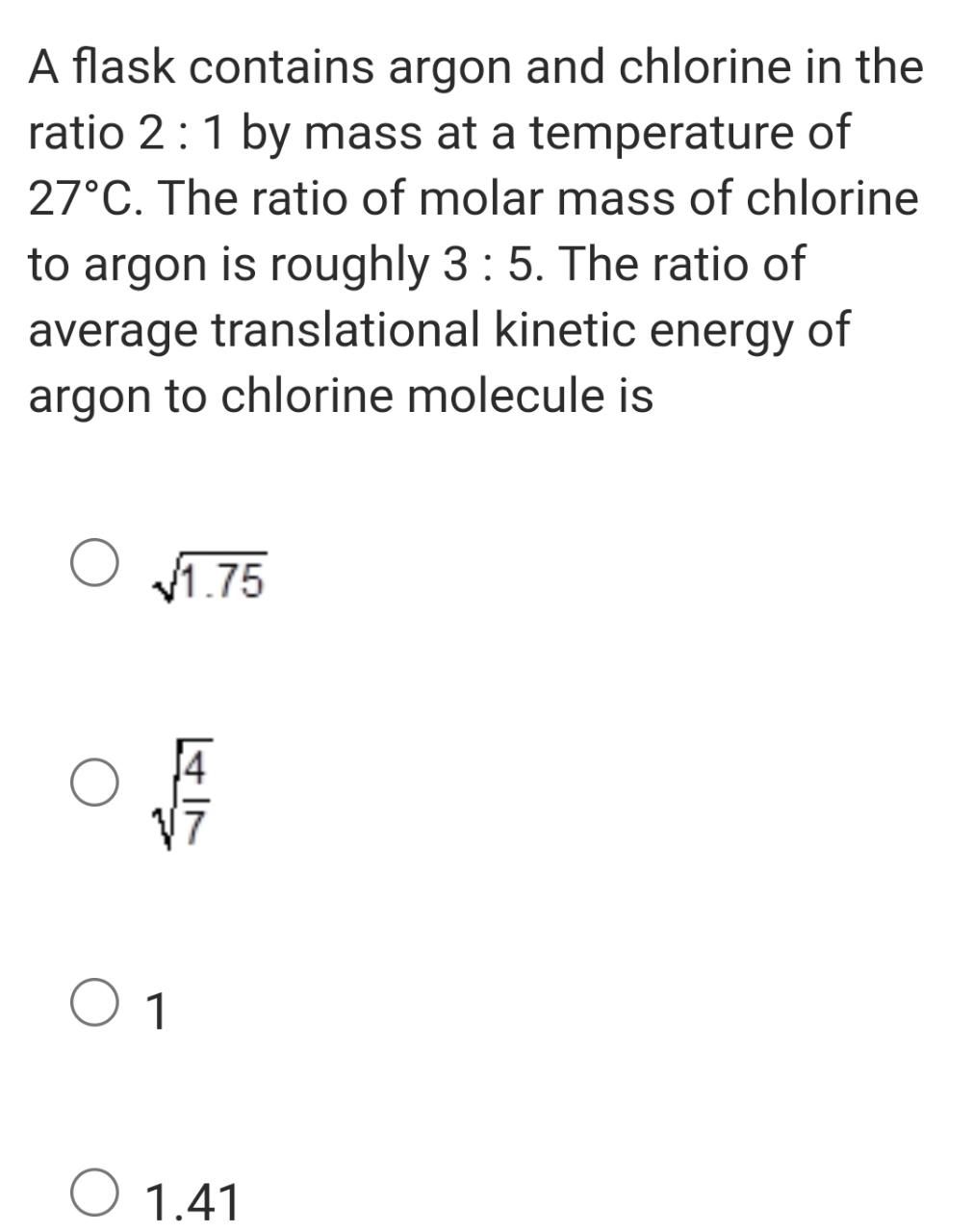
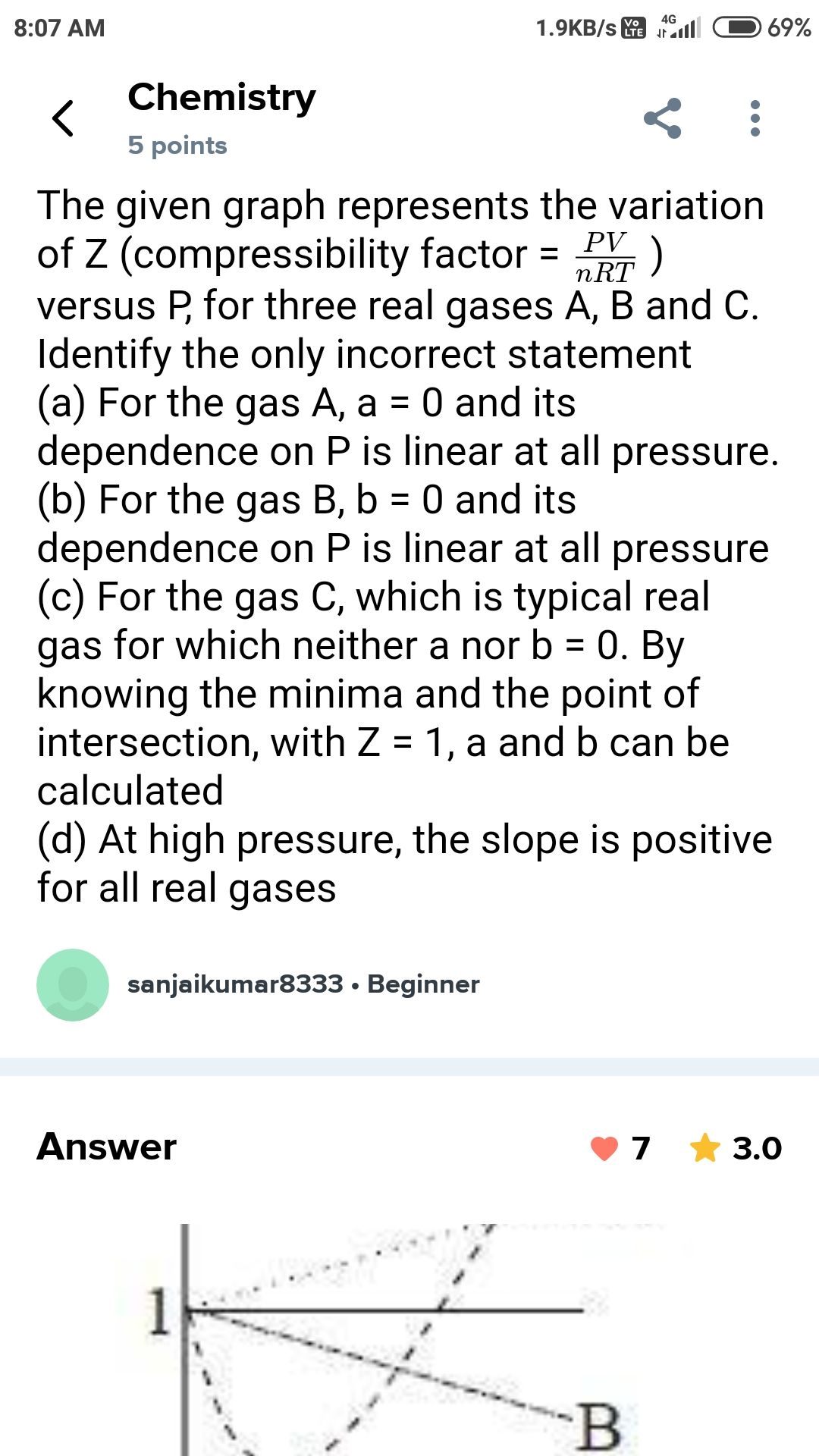
NEET Class neet Answered
please answer this

Asked by Prashant DIGHE | 02 Mar, 2020, 08:50: PM
Given:
t1 for H2 = 5 sec
t2 = ?
We know,
by using Graham's law,
rate of diffusion is inversally proportional to molar mass.
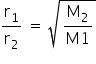
Also we have,
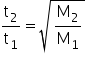
For He,
t2 = 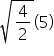
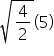
t2 =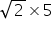 ≠ 10 sec
≠ 10 sec
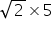 ≠ 10 sec
≠ 10 secFor O2
t2 = 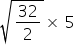
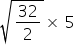
t2 = 20 sec.
For CO
t2 = 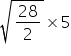
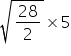
t2 =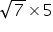
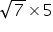
t2 ≠25 s
For CO2
t2 = 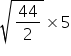
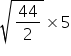
= 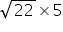
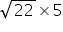
t2 ≠ 55 s
Therefore option (2) is correct.
For O2 : 20 seconds
Answered by Varsha | 03 Mar, 2020, 11:59: AM
NEET neet - Chemistry
Asked by raomayankup83 | 15 Apr, 2024, 07:46: PM
NEET neet - Chemistry
Asked by gopikakannan27 | 13 Jan, 2022, 05:39: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 19 Aug, 2021, 08:38: PM
NEET neet - Chemistry
Asked by kowsalyamouli | 06 Oct, 2020, 04:08: PM
NEET neet - Chemistry
Asked by nssharma001969 | 03 Jun, 2020, 01:54: AM
NEET neet - Chemistry
Asked by prakriti12oct | 25 May, 2020, 12:15: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 10 May, 2020, 08:24: AM
NEET neet - Chemistry
Asked by anushka.kulkarni02022002 | 19 Mar, 2020, 09:31: AM
NEET neet - Chemistry
Asked by Prashant DIGHE | 03 Mar, 2020, 10:09: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 03 Mar, 2020, 10:06: PM