CBSE Class 11-science Answered
please answer this
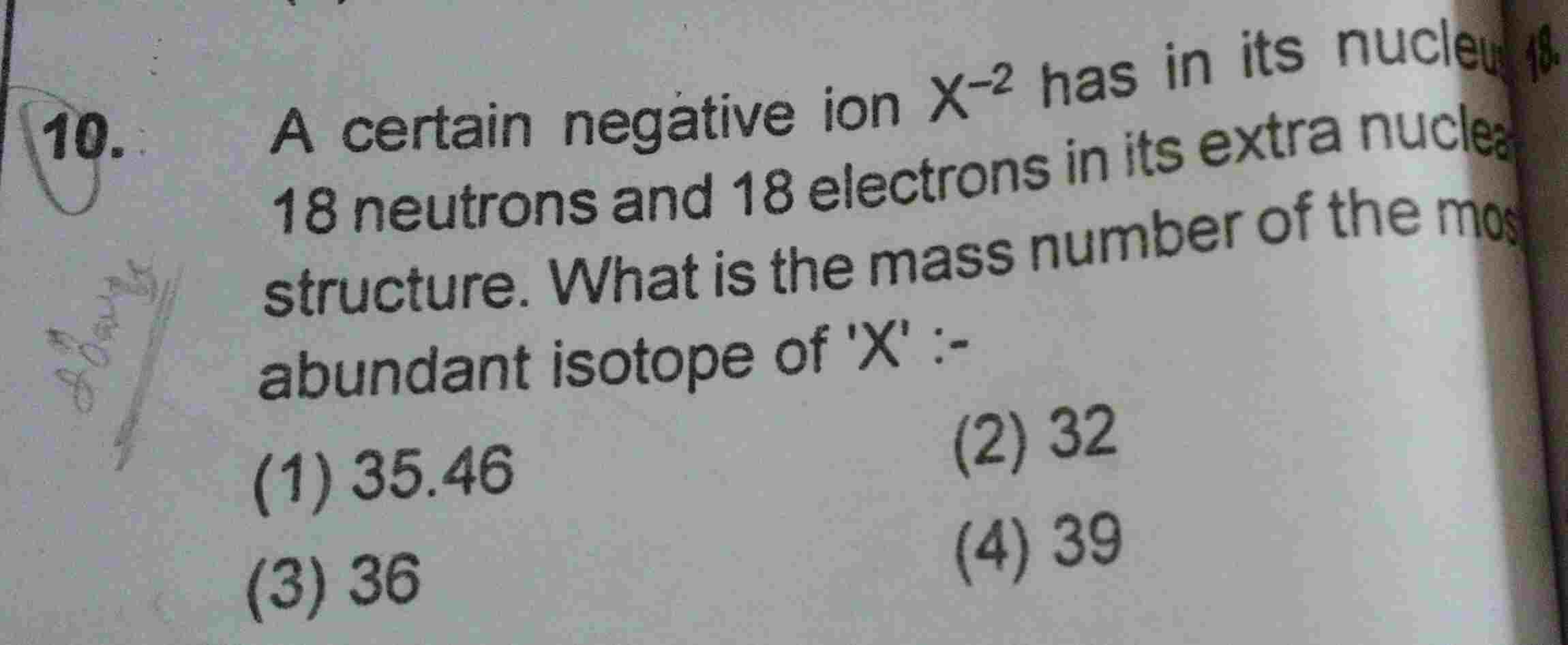
Asked by Prashant DIGHE | 04 Apr, 2020, 10:23: PM
X-2 has 18 e- , Which means X had 16 e- and gained 2 electrons. So total number of electons in X=total number of protons in X=16.
Atomic mass number=16+18=34 u
Atomic niumber=Total number of protons=16
Using periodic table we can say, both 16S34 and 16S32 exist. As 16S32 present in highest proportion we can say it is most abundant isotope.
So, mass number of most abundant isotope of X is =32
Answered by Ravi | 05 Apr, 2020, 06:42: PM
CBSE 11-science - Chemistry
Asked by vishakhachandan026 | 25 May, 2019, 10:17: PM


