NEET Class neet Answered
Please answer the following question with explanation
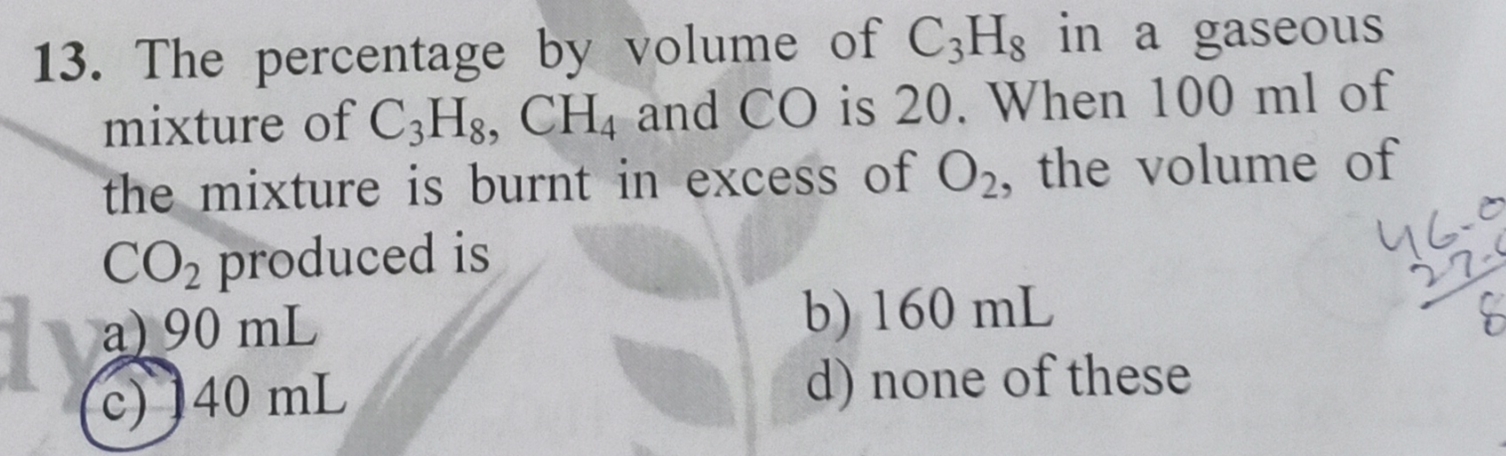
Asked by deepakudgiri29 | 25 Dec, 2018, 12:27: PM
C3H8 CH4 CO
20ml x ml (80-x) ml
C3H8 + 5O2 → 3CO2 + 4H2O
20ml 60ml
CH4 + O2 → CO2 + H2O
x ml x ml
CO + 1/2O2 → CO2
(80-x) 80-x
Total Volume of CO2 released = 60 + x + (80-x) = 140ml. (option c)
Answered by Sumit Chakrapani | 25 Dec, 2018, 03:57: PM
Application Videos
Concept Videos
NEET neet - Chemistry
Asked by dikshitgod07 | 29 Apr, 2024, 08:38: AM
NEET neet - Chemistry
Asked by biswassayan8464 | 21 Apr, 2024, 11:30: AM
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by raomayankup83 | 15 Apr, 2024, 07:46: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
Asked by vasantagomasi23 | 05 Apr, 2024, 08:35: AM