CBSE Class 10 Answered
Is i correct to say that in the reaction between magnesium and oxygen to form magnesium oxide magnesium gets oxidised as it loses electrons and oxygen gets reduced as it loses electrons?
Asked by Viswanath | 23 Sep, 2013, 06:01: PM
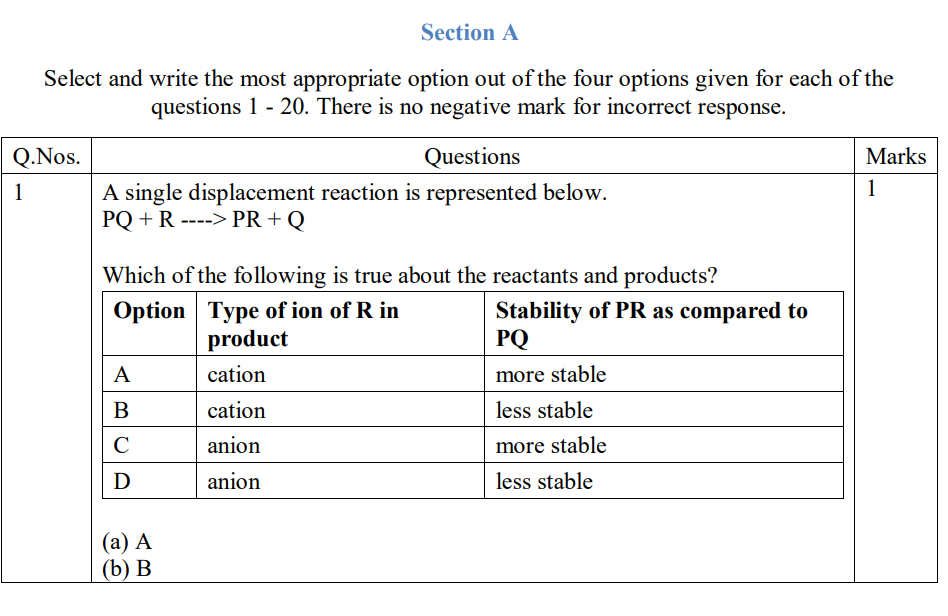
Yes. The reaction between magnesium metal and oxygen to form magnesium oxide involves the oxidation of magnesium.
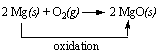
When magnesium reacts with oxygen, each magnesium atom gives two electrons to oxygen. The positive magnesium ions (Mg2+) then combine with negative oxygen ions (O2-) to form solid magnesium oxide (MgO). In this reaction, magnesium (the reducing agent) is oxidized, and oxygen (the oxidizing agent) is reduced.
In the course of this reaction, each magnesium atom loses two electrons to form Mg 2+ ion.
Mg ![]() Mg2+ + 2 e-
Mg2+ + 2 e-
And, each O 2 molecule gains four electrons to form a pair of O 2- ions.
O2 + 4 e- ![]() 2 O2-
2 O2-
Answered by Hanisha Vyas | 23 Sep, 2013, 06:36: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by sagarmishra | 04 Mar, 2024, 09:50: AM
CBSE 10 - Chemistry
Asked by 09.10bjanvhijadhav | 02 Mar, 2024, 08:22: AM
CBSE 10 - Chemistry
Asked by prassanna.j | 01 Mar, 2024, 11:59: AM
CBSE 10 - Chemistry
Asked by yadavparmit83 | 01 Dec, 2023, 06:16: AM
CBSE 10 - Chemistry
Asked by susrisangita792 | 17 Nov, 2023, 08:27: PM
CBSE 10 - Chemistry
Asked by ramjilal01071988 | 14 Oct, 2023, 08:42: PM
CBSE 10 - Chemistry
Asked by dikshantnaik1008 | 21 Jul, 2023, 11:47: AM
CBSE 10 - Chemistry
Asked by susrisangita792 | 07 Jun, 2023, 11:54: AM
CBSE 10 - Chemistry
Asked by shoaibhakak41 | 18 Jan, 2023, 02:17: PM
CBSE 10 - Chemistry
Asked by nehashekh291 | 03 Jan, 2023, 03:11: PM