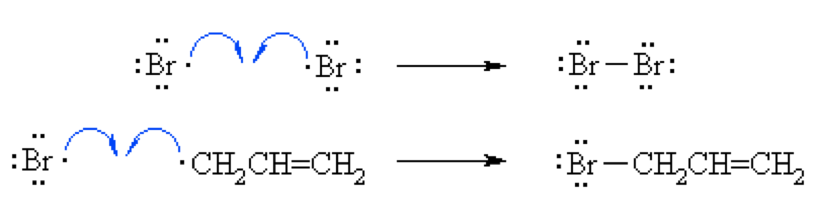CBSE Class 12-science Answered
Let us see the formation mechanism of Allyl bromide.
Step 1(Initiation):
In the presence of heat or UV light, the weak halogen bond undergoes hemolytic fission to generate two bromine radicals and the chain process starts.
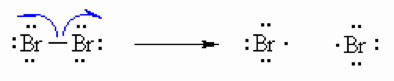
Step 2(Propagation)
The bromine radical abstracts a hydrogen to form HBr and and an allyl radical then the allyl radical abstracts the bromine atom from another bromine molecule to form allyl bromide product and another bromine radical which can then itself undergo reaction creating a cycle that can repeat.
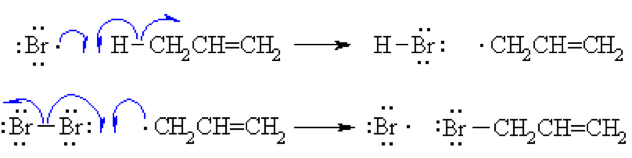
Step 3 (Termination)
Various reactions between the possible pairs of radicals allow for the formation of Br2 or the product allyl bromide. These reactions remove radicals and do not perpetuate the cycle.