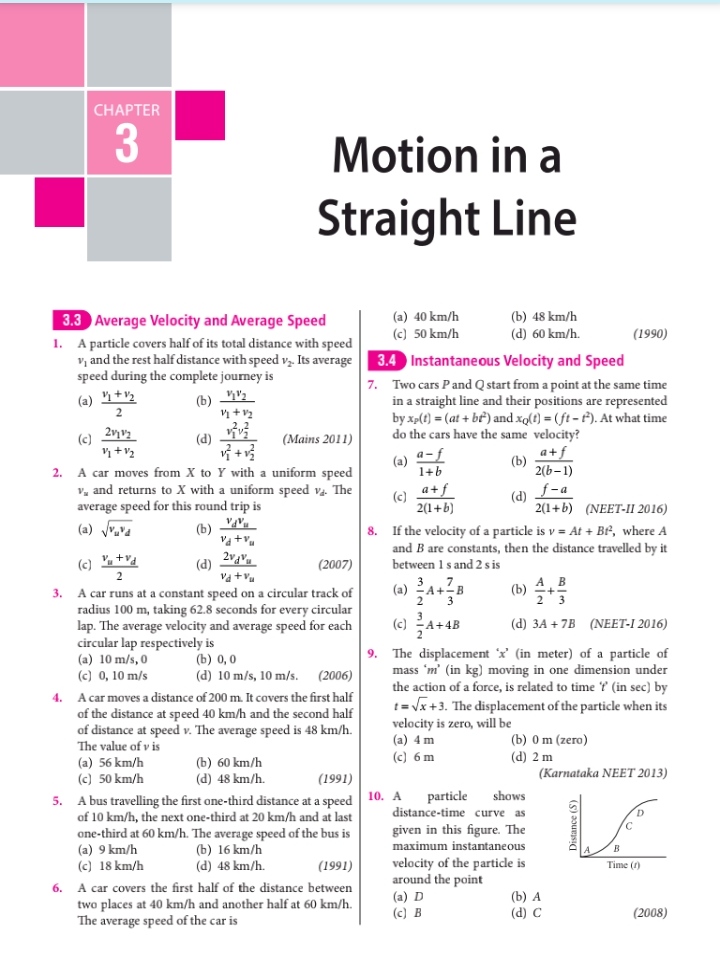
NEET Class neet Answered
For 1 molal aqueous solution of the following compounds, which one will show the highest freezing point ?
(1) [Co(H2O)5Cl]Cl2.H2O
(2) [Co(H2O)4Cl2]Cl.2H2O
(3) [Co(H2O)3Cl3].3H2O
(4) [Co(H2O)6]Cl3
Please explain how to find i for all four and the concept
Asked by nipunverma59 | 09 Jan, 2019, 11:35: PM
We know vant hoff factor, i is directly proportional to depression in freezing point (ΔTf) which is dependent on the number of ions formed in aqueous medium. Here all salts are assumed to be 100% dissociated so their α (degree of dissociation) = 1.
4th salt is supposed to have highest freezing point.
Refer Image for details.
Answered by Sumit Chakrapani | 10 Jan, 2019, 02:39: AM
Application Videos
Concept Videos
NEET neet - Chemistry
Asked by dikshitgod07 | 29 Apr, 2024, 08:38: AM
NEET neet - Chemistry
Asked by biswassayan8464 | 21 Apr, 2024, 11:30: AM
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by raomayankup83 | 15 Apr, 2024, 07:46: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
Asked by vasantagomasi23 | 05 Apr, 2024, 08:35: AM