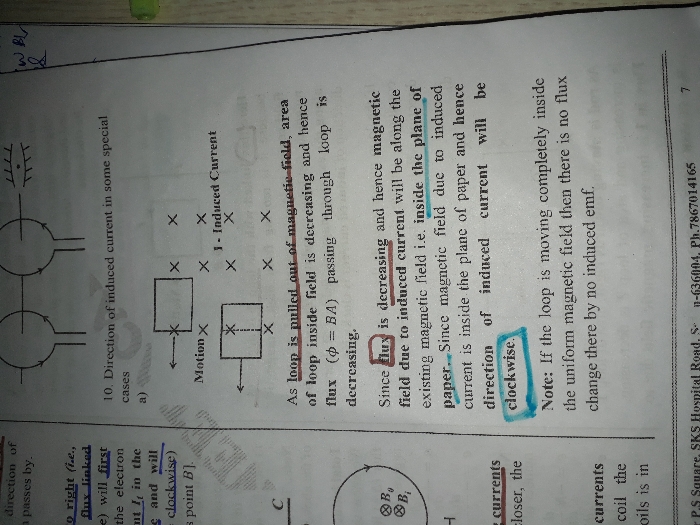
CBSE Class 12-science Answered
find the number of electrons present in an 8g gold pin. given - molar mass of gold = 197g/mol , electrons per atom in gold = 79 and avogadro's number = 6.023*10 23 g-1 mol-1.
Asked by malhanmuskan2003 | 24 Jun, 2020, 02:12: PM

Answered by Utkarsh Lokhande | 24 Jun, 2020, 02:38: PM
Concept Videos
CBSE 12-science - Physics
Asked by ankush76728 | 06 May, 2024, 04:52: PM
CBSE 12-science - Physics
Asked by ankush76728 | 05 May, 2024, 09:55: PM
CBSE 12-science - Physics
Asked by heymindurownbusiness | 04 May, 2024, 11:15: AM
CBSE 12-science - Physics
Asked by talulu | 01 May, 2024, 05:14: PM
CBSE 12-science - Physics
Asked by kanishkg511 | 30 Apr, 2024, 07:25: PM
CBSE 12-science - Physics
Asked by sahoobanita89 | 30 Apr, 2024, 05:10: AM
CBSE 12-science - Physics
Asked by divakar.9124 | 27 Apr, 2024, 10:42: PM
CBSE 12-science - Physics
Asked by panneer1766 | 24 Apr, 2024, 01:52: PM
CBSE 12-science - Physics
Asked by artabandhusahu85 | 24 Apr, 2024, 12:07: PM