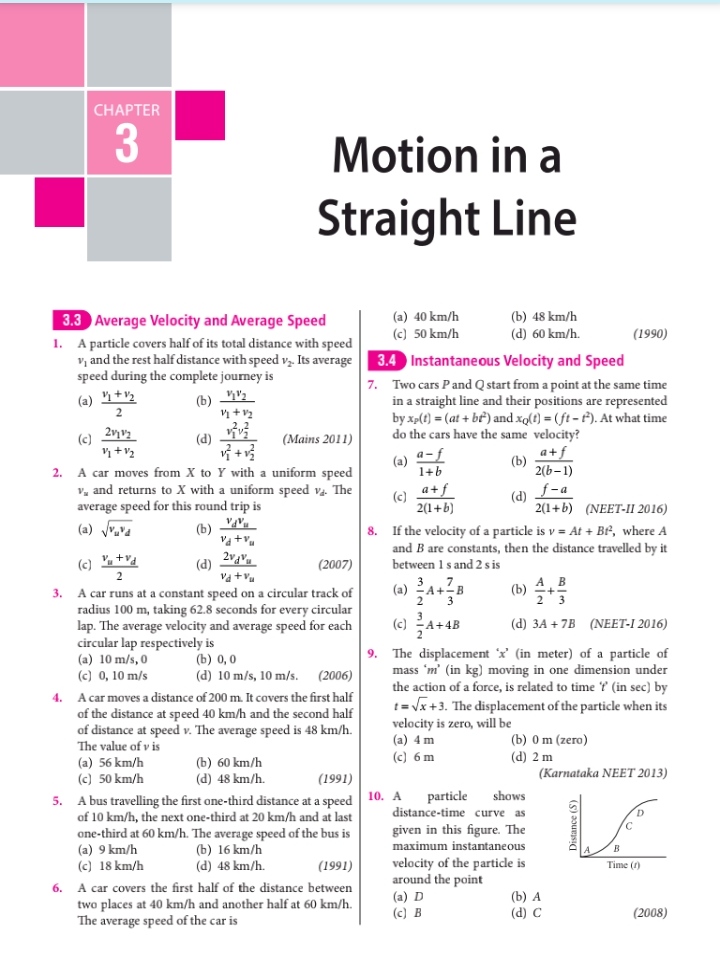
NEET Class neet Answered
explain
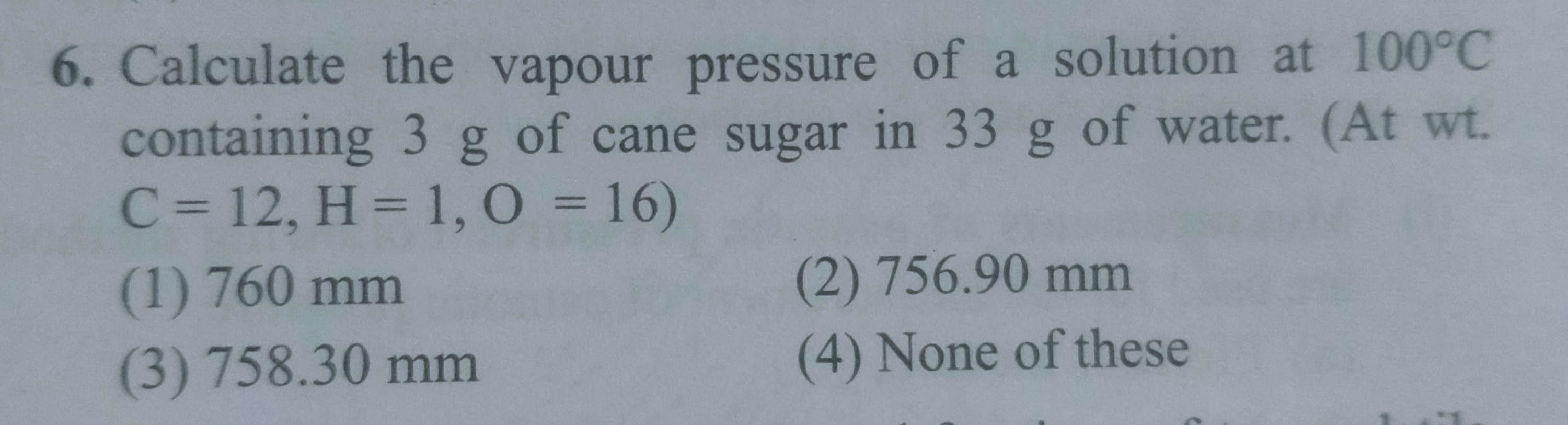
Asked by srushtimahajan125 | 03 Jan, 2023, 06:14: PM
Dear Student,
Vapour pressure of pure water (solvent) at100°C, po = 760 mm
Vapour pressure of solution, p = ?
Wt. of solvent, W = 33 g
Wt. of solute, w = 3 g
Mol. wt. of water = 18
Mol. wt. of sugar (C12H22O11) = 342
According to Raoult’s law,
p° − p / p° = wM / WM
p = p° − (wM/WM) × p°
P = 760 – [(3×18 / 342×33) ×760]
P = 756.9 mm Hg
Hence, correct answer option is 'b'.
Answered by | 04 Jan, 2023, 05:32: PM
Application Videos
Concept Videos
NEET neet - Chemistry
Asked by dikshitgod07 | 29 Apr, 2024, 08:38: AM
NEET neet - Chemistry
Asked by biswassayan8464 | 21 Apr, 2024, 11:30: AM
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by raomayankup83 | 15 Apr, 2024, 07:46: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
Asked by vasantagomasi23 | 05 Apr, 2024, 08:35: AM