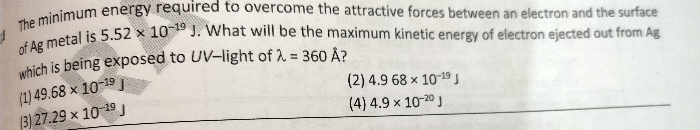CBSE Class 11-science Answered
calculate the number of atoms in 80u of Ne
Asked by swetalinasamantaray022 | 29 Apr, 2023, 08:54: AM
Dear Student,
80 u of Neon contain = 80 / 20 = 4 atoms of neon
(atomic mass of Neon = 20 u)
Answered by | 29 Apr, 2023, 05:58: PM
Application Videos
Concept Videos
CBSE 11-science - Chemistry
Asked by r84314179 | 10 May, 2024, 09:04: AM
CBSE 11-science - Chemistry
Asked by mohd.arhaan812 | 06 May, 2024, 09:55: PM
CBSE 11-science - Chemistry
Asked by r84314179 | 06 May, 2024, 02:28: PM
CBSE 11-science - Chemistry
Asked by shreekrishnampatil | 27 Apr, 2024, 10:31: PM
CBSE 11-science - Chemistry
Asked by luvs6482 | 27 Apr, 2024, 08:18: PM
CBSE 11-science - Chemistry
Asked by gklakshmi701 | 27 Apr, 2024, 09:36: AM
CBSE 11-science - Chemistry
Asked by saranyachakraborty2007 | 25 Apr, 2024, 05:23: AM
CBSE 11-science - Chemistry
Asked by rhythmdraco42 | 22 Apr, 2024, 10:43: PM
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM