CBSE Class 10 Answered
(a) Carbon has 4 electrons in its valence shell. To attain stability, it should either gain 4 electrons or lose 4 electrons. It cannot lose 4 electrons as it involves a lot of energy. Also, it cannot gain 4 electrons because the nucleus cannot hold on to the four extra electrons added. Therefore, to complete the octet, it shares 4 electrons with other atoms. That is why, carbon forms compounds mainly by covalent bonding.
(b) Covalent compounds have covalent bonding in them. The bonds are formed by sharing of electrons. There are no ions in such compounds. There are weak forces of attraction between the molecules. So, they have low melting and boiling points.
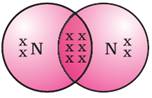
(c) (i) N2
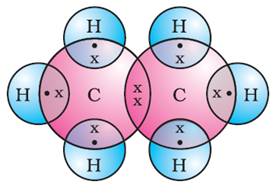
(ii) C2H6
(d) Hex-1-yne
Or
(a) Covalent compounds involve covalent bonding. There are no ions in the covalent compounds. Hence they are poor conductors of electricity.
(b) Carbon dioxide gas is evolved.
It turns lime water milky.
(c) Structural isomers of pentane