CBSE Class 11-science Answered
Copper is extracted from its sulphide ore by following process-
Roasting of sulphide ore:
2Cu2S(s) + 3O2(s) → 2Cu2O(s) + 2SO2(g)
Reduction of copper (I) oxide with copper (I) sulphide:
2Cu2O + Cu2S → 6Cu(s) + SO2(g)
This reaction is known as auto - reduction.
Reaction for electrolytic refining:
At anode : Cu2+(aq) + 2e- → Cu(s)
At cathode : Cu(s) → Cu2+(aq)+ 2e-
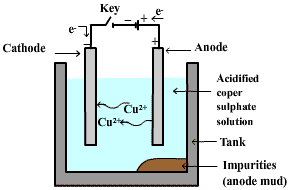
Electrolytic Refining-
In the process of electrolytic refining of copper, the electrolyte used is acidified copper sulphate.
Impure metal is made as an anode and pure metal is made as a cathode.
On passing the electric current through the electrolyte, the impure metal from anode dissolves into the electrolyte solution
and pure metal from copper sulphate solution deposits on the cathode.
The soluble impurities go into the solution and insoluble impurities settle down at the bottom of an anode and are known as anode mud.