ICSE Class 10 Answered
The action of Soap:
Soaps are cleansing agents capable of reacting with water and dislodging the unwanted particles from clothes or skin.
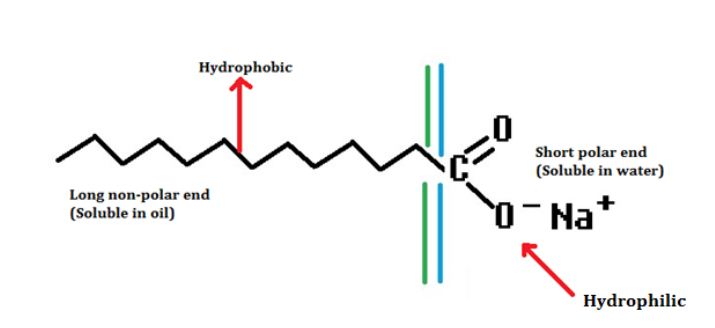
The molecules of soap are sodium or potassium salts of long chain carboxylic acids.
A soap molecule has a tadpole-shaped structure.
At one end (long non-polar end) of the soap molecule is a hydrocarbon chain which is insoluble in water but soluble in oil.
At the other end (short polar end) of soap molecule, there is a carboxylate ion which is hydrophilic i.e. water soluble but insoluble in oil.
Soap on mixing with water forms a concentrated solution and causes foaming.
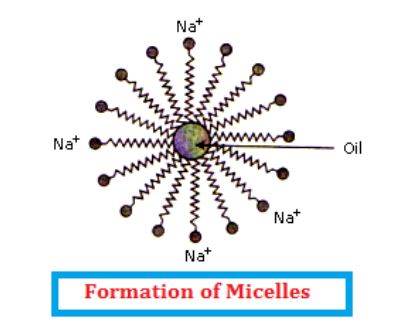
The long non-polar end of soap gravitates towards and surrounds the dirt and absorbs the dust in it.
The short polar end with the carboxylate ion repels the water away from the dirt.
A spherical aggregate of soap molecules is formed in the soap solution in water and is called a micelle.
Thus, the soap molecule dissolves the dirt and our clothes get clean.