JEE Class main Answered
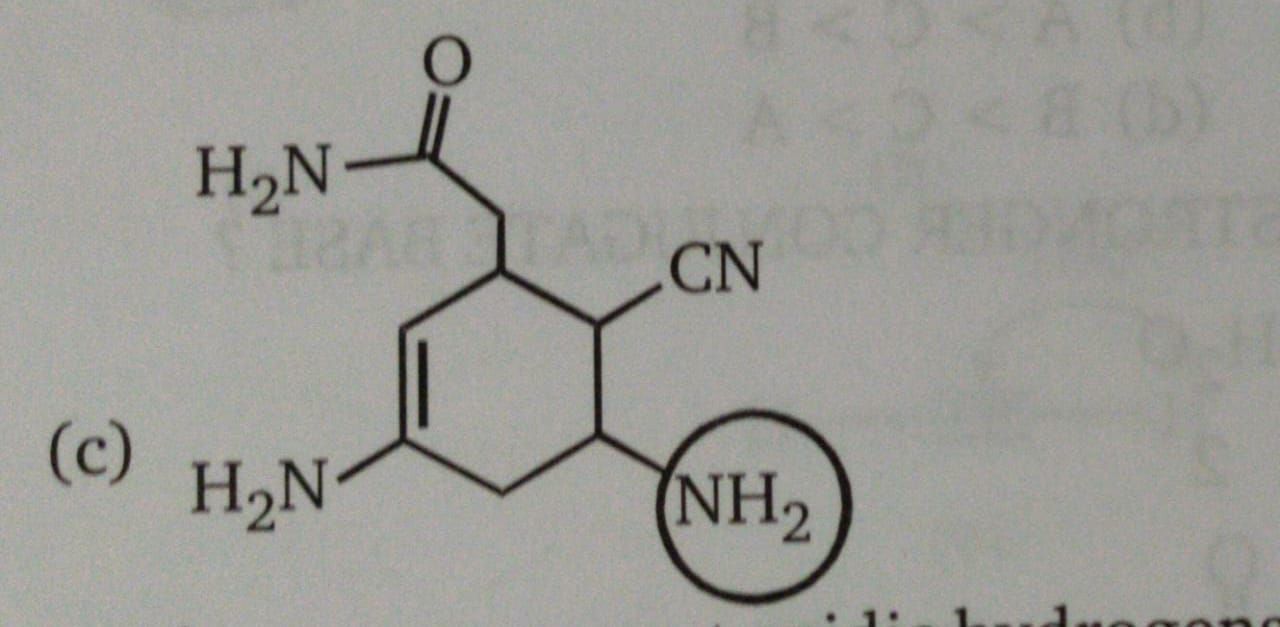
Why is the circled Nitrogen most basic in this case?
Asked by Mrinal | 31 Aug, 2019, 15:00: PM
In the given compound, one –NH2 group is in conjugation with carbon-carbon double bond and other is with a carbonyl group. Hence, the unshared pair of electrons on nitrogen is less available for protonation because of resonance.
The –NH2 group which is circled in the given compound shows no resonating structure which suggests that its lone pair is available for a donation.
Therefore the circled amine group is most basic than rest of groups.
Answered by Ramandeep | 03 Sep, 2019, 13:26: PM
JEE main - Chemistry
Asked by netrapalsaini71 | 06 Jan, 2024, 15:45: PM
JEE main - Chemistry
Asked by Mrinal | 31 Aug, 2019, 15:00: PM
JEE main - Chemistry
Asked by sumayiah2000 | 29 Apr, 2019, 11:42: AM