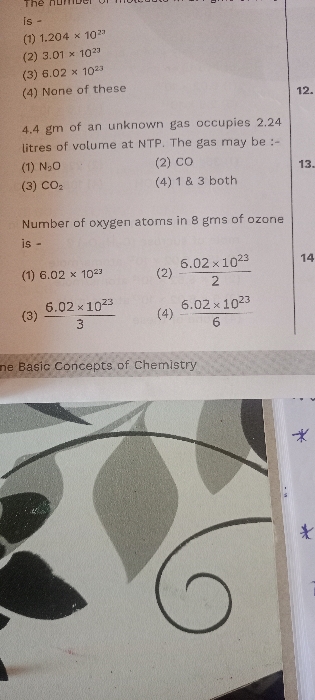NEET Class neet Answered
Which of the following statement is correct?
A) 28g of CO contains 12g of carbon and 16g oxygen
B)one mole of CO reacts completely with half mole of O2
C)N2 and CO have same molar mass
D) all of these

Asked by vrishti.8416 | 24 Feb, 2022, 03:30: PM
All of these statements are correct. Please check one by one.
(a) Molar mass of CO= Atomic mass of C + Atomic mass of O
= 12+16=28 g
So, We can say- 28g of CO contains 12g of carbon and 16g oxygen .
(b) Let's write balanced chemical equation when CO reacts with O2
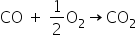
So, from the stoichiometry of this balanced equation
We can say one mole of CO reacts completely with half mole of O2.
(c) 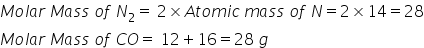
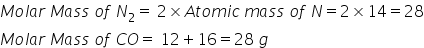
Answered by Ravi | 24 Feb, 2022, 05:54: PM
NEET neet - Chemistry
Asked by biswassayan8464 | 21 Apr, 2024, 11:30: AM
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
Asked by vaka.aruna1979 | 23 Mar, 2024, 04:18: AM
NEET neet - Chemistry
Asked by fathimahusna23042004 | 03 Mar, 2024, 08:56: AM
NEET neet - Chemistry
Asked by drkeshavkhandagle | 18 Jan, 2024, 08:10: PM
NEET neet - Chemistry
Asked by yogitakumawat | 21 Dec, 2023, 10:31: PM