JEE Class main Answered
What volume of a solution of Hcl containing 73g/litre would suffice for the exact nuetralisation of NaOH obtained by allowing 0.46g of metallic sodium to react with water?
Asked by goyatvanshika55 | 15 Jun, 2019, 18:36: PM
The reactions are
Na + H2O → NaOH + 1/2 H2
NaOH + HCl → NaCl + H2O
Meq of HCl = Meq of NaOH = Meq of Na
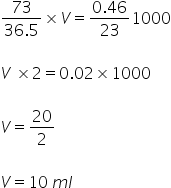
Volume required is 10 ml
Answered by Varsha | 16 Jun, 2019, 08:54: AM
JEE main - Chemistry
Asked by Mdizhanshaikh | 20 May, 2024, 19:18: PM
JEE main - Chemistry
Asked by manishguptaballia.15 | 11 May, 2024, 15:57: PM
JEE main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 17:37: PM
JEE main - Chemistry
Asked by gmafia618 | 04 Apr, 2024, 20:48: PM
JEE main - Chemistry
Asked by jadhavshivtej256 | 27 Feb, 2024, 18:25: PM
JEE main - Chemistry
Asked by pradumankumarsah1 | 30 Jan, 2024, 14:36: PM
JEE main - Chemistry
Asked by srujan11042008 | 06 Nov, 2023, 10:31: AM





