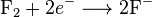CBSE Class 10 Answered
A good example is the reaction between hydrogen and fluorine:
We can write this overall reaction as two half-reactions: the oxidation reaction
and the reduction reaction:
Analysing each half-reaction in isolation can often make the overall chemical process clearer. Because there is no net change in charge during a redox reaction, the number of electrons in excess in the oxidation reaction must equal the number consumed by the reduction reaction (as shown above).
Elements, even in molecular form, always have an oxidation number of zero. In the first half reaction, hydrogen is oxidized from an oxidation number of zero to an oxidation number of +1. In the second half reaction, fluorine is reduced from an oxidation number of zero to an oxidation number of −1.
When adding the reactions together the electrons cancel: