CBSE Class 10 Answered
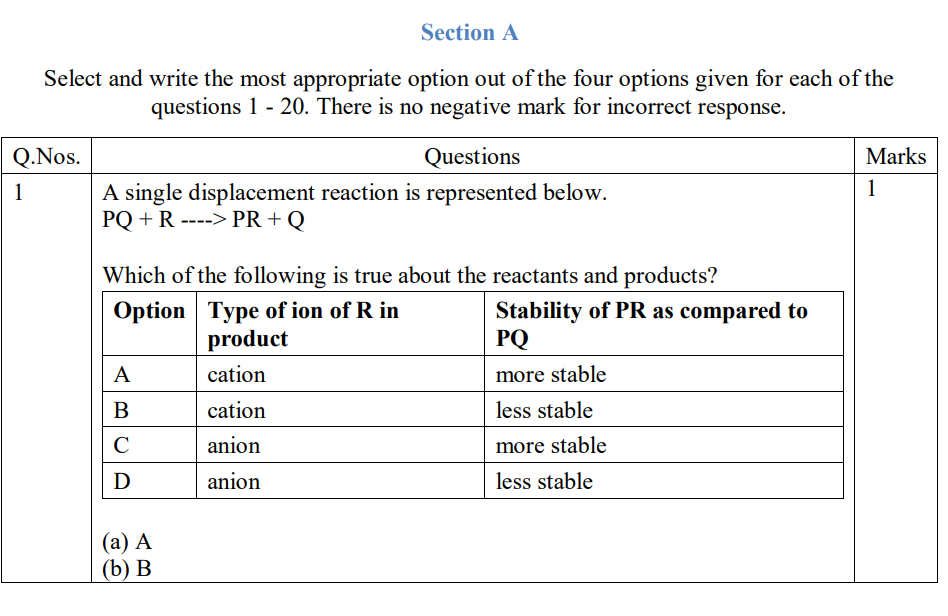
Metallic atom loses electrons to attain stable electronic configuration and become a cation.
e.g. Na ![]() Na+
Na+
2, 8, 1 2, 8
(Neutral) (Cation)
Non-metallic atom gains electrons to attain stable electronic configuration and become an anion.
e.g., Cl ![]() Cl–
Cl–
2, 8, 7 2, 8, 8
(Neutral) (Anion)
Cations and anions are oppositely charged particles, which attract one another to form an electrovalent bond leading to the formation of an electrovalent compound.
Formation of Sodium Chloride
1] Ionic equation
Na - 1e- → Na+
(2, 8, 1) (2, 8)
Cl + 1e- → Cl-
(2, 8, 7) (2, 8, 8)
___________________________________
Na + Cl → Na+ Cl- → NaCl