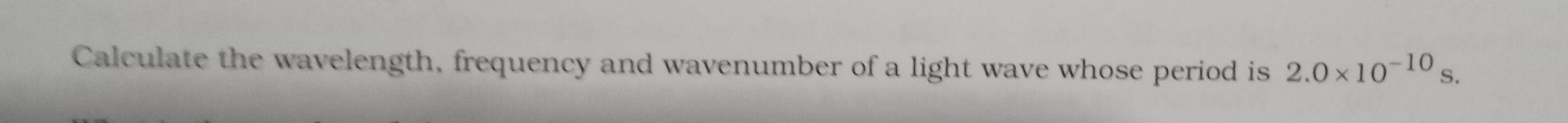JEE Class main Answered
The dissociation energy of H2 is 430.53 k J rnol-1 . If H2 is exposed to radiation energy of wavelength 253.7 nm, what % of radiant energy will be converted into kinetic energy?
Asked by eggwallet18 | 28 Apr, 2019, 21:29: PM
Given:
The dissociation energy of H2 = 430.53 kJ/mol
= 430.53× 103 J/mol
Wavelength=253.7 nm
= 253.7 ×10-9 m
Planck's constant, h = 6.626×10−34 Jsec
To break H-H bond energy reaquired is,

Energy of photon =
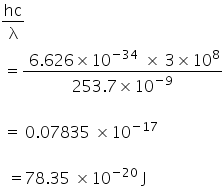
Energy converted to kinetic energy =
78.35 ×10−20 − 71.49 ×10−20 J
= 6.86 ×10−20 J
% of radiant energy converted into kinetic energy,
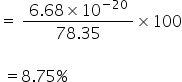
8.75% of radiant energy will be converted into kinetic energy.
Answered by Varsha | 29 Apr, 2019, 11:59: AM
JEE main - Chemistry
Asked by radham6375 | 17 May, 2024, 20:13: PM
JEE main - Chemistry
Asked by 9079344910choudhary | 16 May, 2024, 18:52: PM
JEE main - Chemistry
Asked by purnendurai26 | 02 May, 2024, 18:34: PM
JEE main - Chemistry
Asked by cheekatiyogendra143 | 20 Apr, 2024, 11:16: AM
JEE main - Chemistry
Asked by jwhhebbb | 19 Apr, 2024, 13:21: PM
JEE main - Chemistry
Asked by adityadoodi3 | 05 Apr, 2024, 23:27: PM
JEE main - Chemistry
Asked by pratap62437 | 19 Feb, 2024, 12:48: PM
JEE main - Chemistry
Asked by sayushman087 | 01 Feb, 2024, 10:28: AM
JEE main - Chemistry
Asked by marthalamanoharreddy65 | 17 Dec, 2023, 10:26: AM