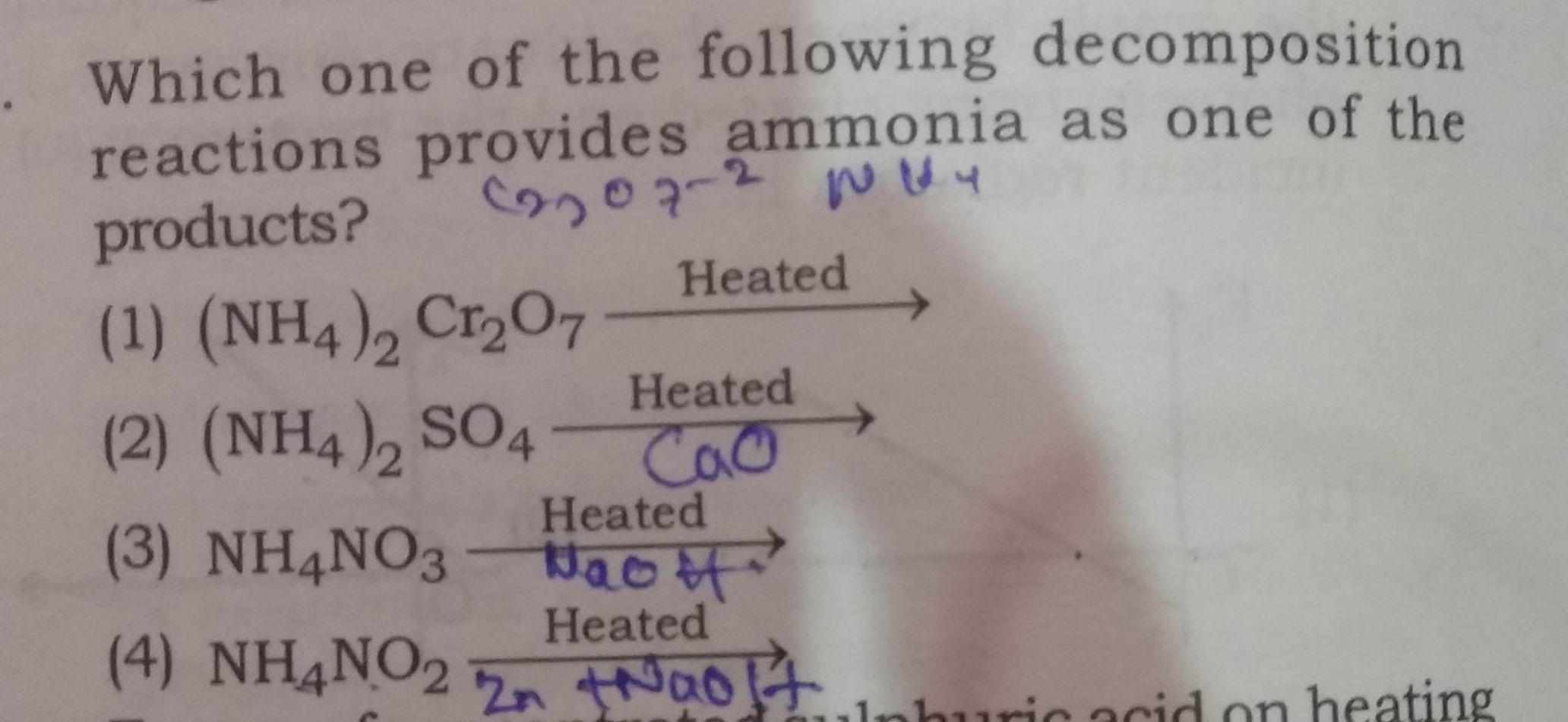
NEET Class neet Answered
With increase in oxidation number of a particular halogen atom, the acidic character of corresponding oxoacid increases.
According to Lowry-Bronsted concept, a strong acid has weak conjugate base and a weak acid has a strong conjugate base. Now, let us consider the stabilities of the conjugate bases ClO4-, ClO3-, ClO2- bases, ClO formed from these acids, HClO, HClO2, HClO3 and HClO4 respectively. These anions are stabilised to greater extent, it has lesser attraction for proton and therefore, will behave as weaker base. Consequently, the corresponding acid will be strongest because weak conjugate base has strong acid and strong conjugate base has weak acid and vice versa.
Now the charge stabilization will be minimum. The charge stabilization increases in the order: ClO- < -ClO2- < -ClO3- < -ClO4-. This means that ClO- will have minimum stability and therefore, will have maximum attraction for H+. Thus, ClO- will be strongest base and so its conjugate acid HClO will be the weakest acid. Similarly, in this series ClO4- is the weakest base (maximum stabilized) and its conjugate acid HClO4 is the strongest acid. Hence, the acidic strength increases in the order: HClO < HClO2 < HClO3 < HClO4.