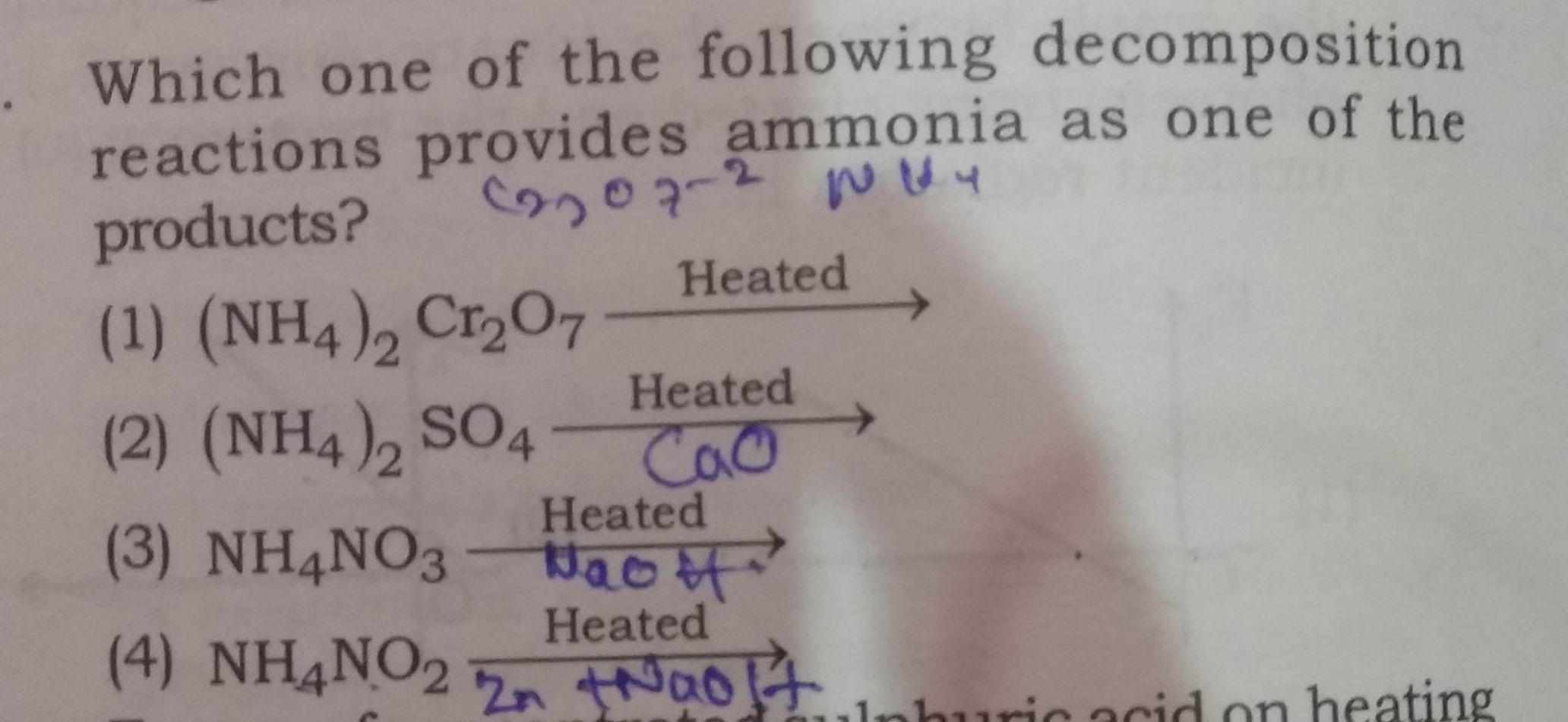
NEET Class neet Answered
pcl6- exist kyu nhi krta hai??
Asked by abdulrazz0088 | 02 Jan, 2024, 08:47: PM
Dear Student,
In the case of phosphorous, the outermost electronic configuration is [Ne]3s23p3
The reason for the valency of 3 is that it needs three more electrons to complete its octet.
The transfer of an electron from a 3s orbital into a 3d orbital accounts for the valency of 5. As a result, five sp3d hybrid orbitals are formed, giving phosphorous a valency of 5.
Thus, phosphorous can form 5 bonds. Hence, PCl6 does not exist.
Answered by | 03 Jan, 2024, 10:18: AM
Concept Videos
NEET neet - Chemistry
Asked by a9460552973 | 02 Mar, 2024, 07:42: AM
NEET neet - Chemistry
Asked by abdulrazz0088 | 02 Jan, 2024, 08:47: PM
NEET neet - Chemistry
Asked by sayesha7862026 | 02 Nov, 2023, 06:32: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 18 Oct, 2020, 04:57: PM
NEET neet - Chemistry
Asked by arnavvidudala20050 | 16 Apr, 2020, 10:11: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 12 Apr, 2020, 03:37: AM
NEET neet - Chemistry
Asked by Prashant DIGHE | 07 Apr, 2020, 09:53: PM