JEE Class main Answered
Sir plz solve it step by step
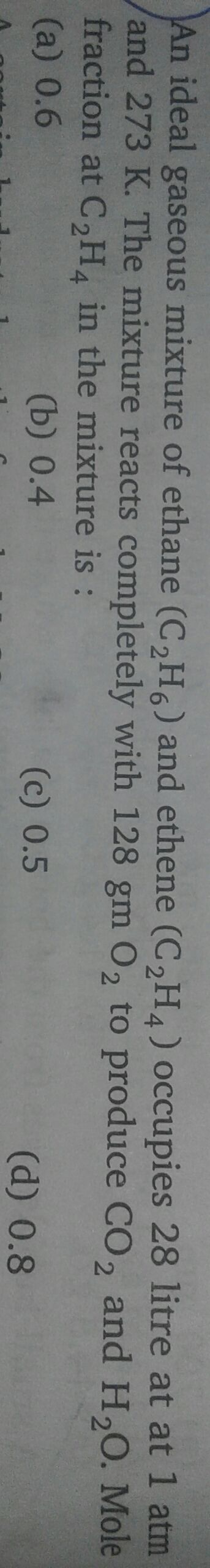
Asked by vishakhachandan026 | 26 Jul, 2019, 15:05: PM
Given:
Volume of mixture of gases = 28 L
Pressure = 1atm
Temperature = 273 K
Using gas equation,
PV = nRT
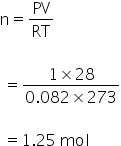
let C2H4 = x
C2H6 = y
x + y = 1.25 mol ....(1)
The mixture of gases reacts with O2
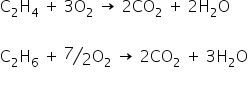
Mixture completely reacts with 128 gm of O2
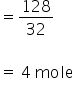
Now,
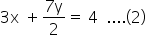
From eq (1) and (2)
y= 0.5 mol
Mole fraction of C2H4
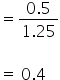
So mole fraction of C2H4 = 1- 0.4
= 0.6
Answered by Varsha | 26 Jul, 2019, 17:34: PM
JEE main - Chemistry
Asked by Mdizhanshaikh | 20 May, 2024, 19:18: PM
JEE main - Chemistry
Asked by manishguptaballia.15 | 11 May, 2024, 15:57: PM
JEE main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 17:37: PM
JEE main - Chemistry
Asked by gmafia618 | 04 Apr, 2024, 20:48: PM
JEE main - Chemistry
Asked by jadhavshivtej256 | 27 Feb, 2024, 18:25: PM
JEE main - Chemistry
Asked by pradumankumarsah1 | 30 Jan, 2024, 14:36: PM
JEE main - Chemistry
Asked by srujan11042008 | 06 Nov, 2023, 10:31: AM





