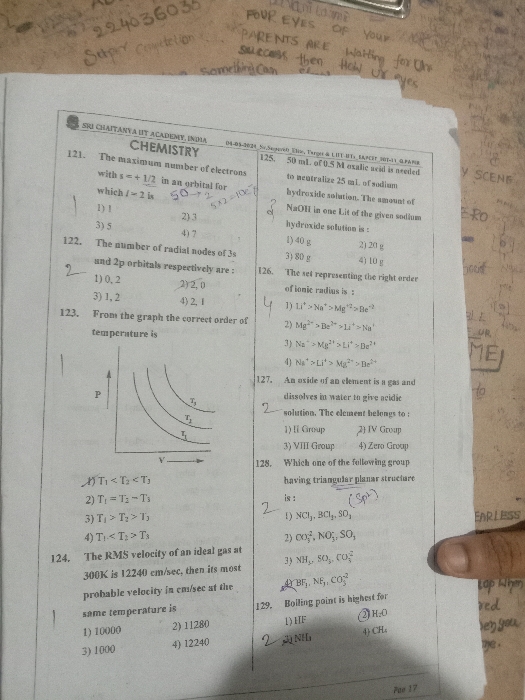
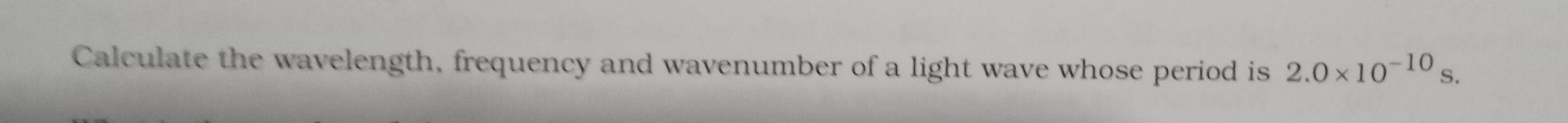JEE Class main Answered
Sir please solve and explain me the concept in Q.6.
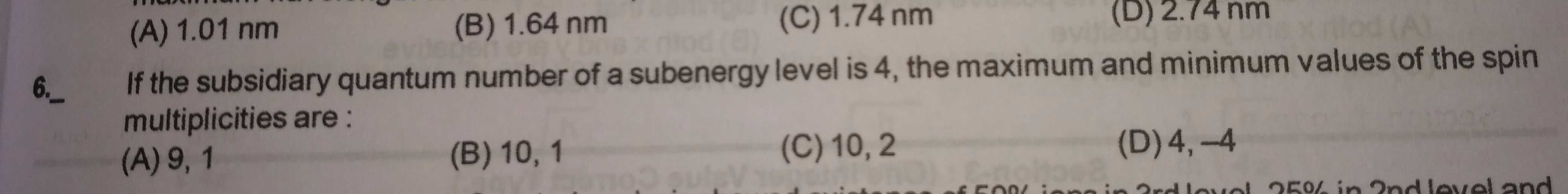
Asked by Joydeep | 14 Jul, 2019, 12:51: PM
The spin multiplicity value =2S + 1. Where, S is the sum of spins of the electrons.
Subsidiary quantum number of 4 indicates g orbital with 9 degenerate sub- orbitals. The orbital can take up a maximum of 18 electrons.
Spin of an electron is either +1/2 or -1/2 depending on the if the electron spins in the clockwise or anticlockwise direction.If there is one electron in the g orbital,
S = +1/2For a pair of electrons in a g sub-orbital, the spin quantum number cancels out.So, unpaired electrons effectively contribute to spin multiplicity.The maximum value will be given by 9 unpaired electrons with positive spin.S = 9 ×× ½ = 4.5Multiplicity = 2S + 1 = 2 ×× 4.5 + 1 = 10The minimum value will be given when no unpaired electron is present S = 0
Multiplicity = 2 ×× 0+ 1 = 1
So, maximum and minimum values are 10. 1
Hence option B is correct
Answered by Ravi | 15 Jul, 2019, 19:17: PM
JEE main - Chemistry
Asked by radham6375 | 17 May, 2024, 20:13: PM
JEE main - Chemistry
Asked by 9079344910choudhary | 16 May, 2024, 18:52: PM
JEE main - Chemistry
Asked by purnendurai26 | 02 May, 2024, 18:34: PM
JEE main - Chemistry
Asked by cheekatiyogendra143 | 20 Apr, 2024, 11:16: AM
JEE main - Chemistry
Asked by jwhhebbb | 19 Apr, 2024, 13:21: PM
JEE main - Chemistry
Asked by adityadoodi3 | 05 Apr, 2024, 23:27: PM
JEE main - Chemistry
Asked by pratap62437 | 19 Feb, 2024, 12:48: PM
JEE main - Chemistry
Asked by sayushman087 | 01 Feb, 2024, 10:28: AM
JEE main - Chemistry
Asked by marthalamanoharreddy65 | 17 Dec, 2023, 10:26: AM