JEE Class main Answered
Should we give more priority to the rule that resonance structure with all atoms having octet is more stable ,or, to the rule that more electronegative atom should have negative charge on it and less electronegative atom should have positive charge on it, while deciding between stability of resonance structures? What will be the answer of the questions a and b in the picture then?

Asked by sumayiah2000 | 01 Apr, 2019, 07:53: AM
Here, In this case, you can remember the following key points:
Given compound is always more stable than its resonating structures (Charged species are less stable than uncharged)
No. of covalent increases stability increases.
Unlike charge separation (distance between opposite charges) decreases stability increases while like (distance between same charges) charges increases stability increases.
Negative charged is least stable at the less electronegative atom while it is more stable at a more electronegative atom.
In the given question,
(a) Vinyl chloride:
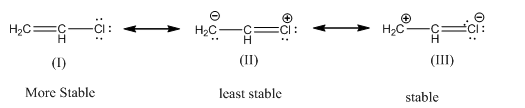
The order of stbility is I > III > II
(b) Formic acid:
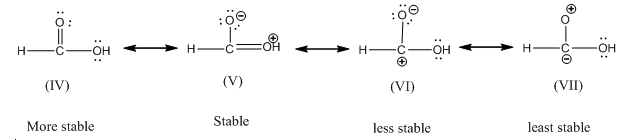
The order of stbility is IV > V > VI > VII
Answered by Ramandeep | 10 Apr, 2019, 02:48: PM
JEE main - Chemistry
Asked by manoradh7322005 | 25 Apr, 2020, 10:40: AM
JEE main - Chemistry
Asked by sumayiah2000 | 01 Apr, 2019, 07:53: AM