JEE Class main Answered
Q.no. :48,plz solve it step by step wih calculations,thanks

Asked by vishakhachandan026 | 28 Jun, 2019, 17:40: PM
Given:
C =70.6%, H=4.2%, N=11.8%, O=13.4%.
Consider, 100 gm of kevlar will have,
C =70.6 gm,
H=4.2 gm,
N=11.8 gm,
O=13.4 gm.
Lets find out the moles of each element, as we know the molar masses,
C=12, H=1, N=14, O=16

Lets divide each with smallest moles i.e., 0.84 mol we get,
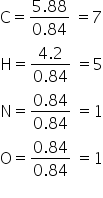
Hence the emperical formula will be C7H5NO
Answered by Ramandeep | 28 Jun, 2019, 18:27: PM
JEE main - Chemistry
Asked by Mdizhanshaikh | 20 May, 2024, 19:18: PM
JEE main - Chemistry
Asked by manishguptaballia.15 | 11 May, 2024, 15:57: PM
JEE main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 17:37: PM
JEE main - Chemistry
Asked by gmafia618 | 04 Apr, 2024, 20:48: PM
JEE main - Chemistry
Asked by jadhavshivtej256 | 27 Feb, 2024, 18:25: PM
JEE main - Chemistry
Asked by pradumankumarsah1 | 30 Jan, 2024, 14:36: PM
JEE main - Chemistry
Asked by srujan11042008 | 06 Nov, 2023, 10:31: AM





