JEE Class main Answered
Q) What will be the maximum numbers of electrons having same spin, present in an atom for n+l=4?
Asked by Anish | 16 Jan, 2019, 00:24: AM
Given:
n + l = 4
let's find the possible values of n and l whose total will be 4
therefore he possible values are n=3 & l=1 and n=4 and l = 0.
For n=3 & l=1
n=3 and l=1 represent the 3p subshell. therefore, the maximum number of electrons in this subshell = 6
For n=4 and l = 0
n=4 and l = 0 represent the 4s subshell. therefore, the maximum number of electrons in this subshell = 2
the total number of electrons = 6 + 2 = 8
Hence the electrons with the same spin = 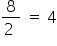
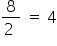
Answered by Ramandeep | 18 Jan, 2019, 12:59: PM
JEE main - Chemistry
Asked by Mdizhanshaikh | 20 May, 2024, 19:18: PM
JEE main - Chemistry
Asked by manishguptaballia.15 | 11 May, 2024, 15:57: PM
JEE main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 17:37: PM
JEE main - Chemistry
Asked by gmafia618 | 04 Apr, 2024, 20:48: PM
JEE main - Chemistry
Asked by jadhavshivtej256 | 27 Feb, 2024, 18:25: PM
JEE main - Chemistry
Asked by pradumankumarsah1 | 30 Jan, 2024, 14:36: PM
JEE main - Chemistry
Asked by srujan11042008 | 06 Nov, 2023, 10:31: AM





