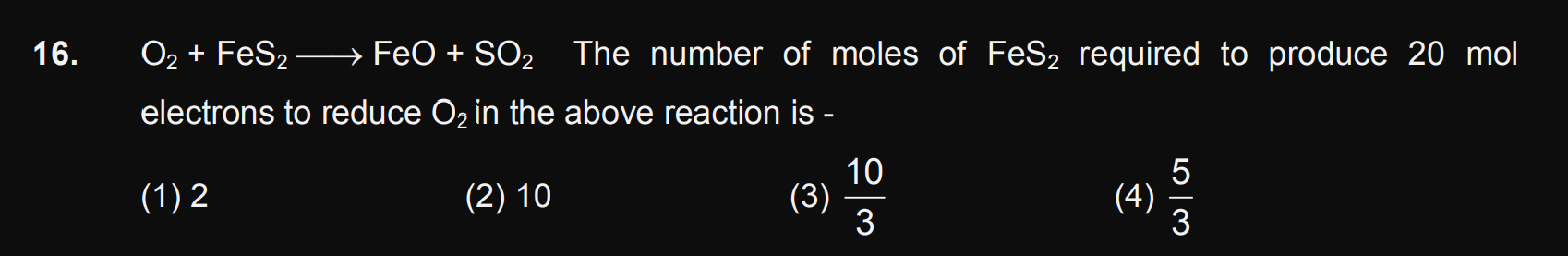JEE Class main Answered
Q)A long rectangular box is filled with Cl2 (atomic mass=35.45) which is known to contain only Cl35 and Cl37. If the box can be divided into a partition and the two types of chlorine molecule are put into two compartments respectively. Calculate where should partition to be made if pressure on both sides is to be same? The ratio of pressure to original pressure is 1/x.
So, the value of x is?
Asked by Anish | 08 Jan, 2019, 23:32: PM
Let us consider in the mixture,
The mole of Cl(35) = n1
The mole of Cl(37) = n2
Average molar mass = 


PV1=n1RT PV2=n2RT
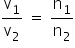
At constant P and T
The number of moles α Volume of gas
Therefore, the partition should be made in the volume ratio of 3.44:1.
Also, as we found that the pressure at this condition is the same as the original condition since the volume of the box and the number of mole along with temperature are constant.
So, the value of x should be 1.
Answered by Ramandeep | 09 Jan, 2019, 11:26: AM
JEE main - Chemistry
Asked by Mdizhanshaikh | 20 May, 2024, 19:18: PM
JEE main - Chemistry
Asked by manishguptaballia.15 | 11 May, 2024, 15:57: PM
JEE main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 17:37: PM
JEE main - Chemistry
Asked by gmafia618 | 04 Apr, 2024, 20:48: PM
JEE main - Chemistry
Asked by jadhavshivtej256 | 27 Feb, 2024, 18:25: PM
JEE main - Chemistry
Asked by pradumankumarsah1 | 30 Jan, 2024, 14:36: PM
JEE main - Chemistry
Asked by srujan11042008 | 06 Nov, 2023, 10:31: AM