JEE Class main Answered
Please solve
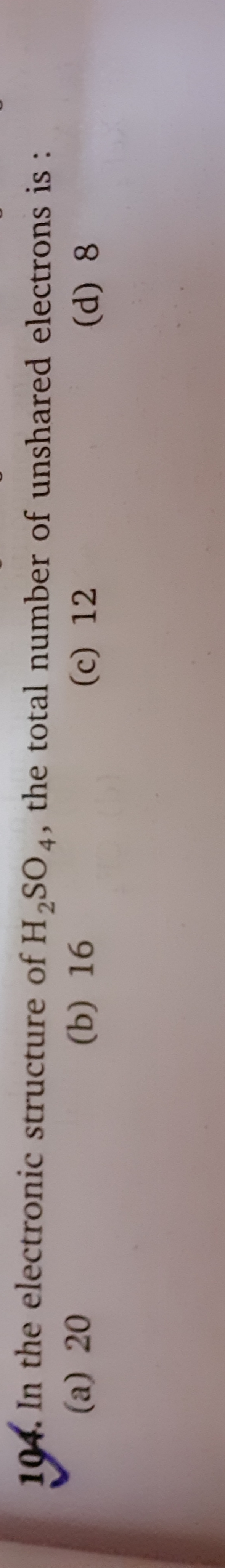
Asked by muditchopra | 11 Jun, 2020, 10:42: AM
The chemical formula of Sulpuric acid is H2SO4:
Sulphur atom (2, 8, 6) is the central atom have six valence electrons, as it has vacant d-orbital sulphur can show variable valencies.
Oxygen atom: 1s2, 2s2, 2p4
Hydrogen atom: 1s1
Total no. of outermost electrons = 6×4+6×1+1×2=32
No. of shared electrons =2×no. of covalent bonds=2×8=16
No. of unshared e−s=32−16=16
Hence, the correct option is A
Answered by Ramandeep | 11 Jun, 2020, 12:35: PM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by hv5594265 | 12 Jun, 2024, 11:59: AM
JEE main - Chemistry
Asked by radham6375 | 17 May, 2024, 20:13: PM
JEE main - Chemistry
Asked by 9079344910choudhary | 16 May, 2024, 18:52: PM
JEE main - Chemistry
Asked by purnendurai26 | 02 May, 2024, 18:34: PM
JEE main - Chemistry
Asked by cheekatiyogendra143 | 20 Apr, 2024, 11:16: AM
JEE main - Chemistry
Asked by jwhhebbb | 19 Apr, 2024, 13:21: PM
JEE main - Chemistry
Asked by adityadoodi3 | 05 Apr, 2024, 23:27: PM
JEE main - Chemistry
Asked by pratap62437 | 19 Feb, 2024, 12:48: PM
JEE main - Chemistry
Asked by malayakdha | 07 Feb, 2024, 12:30: PM