CBSE Class 10 Answered
Please explain oxidation and reduction in terms of loss or gain of electrons with examples
Asked by Trisha Gupta | 24 Sep, 2021, 15:04: PM
- Oxidation is the loss of electrons and reduction is the gain of electrons.
- To explain the involvement of oxidation and reduction in the formation of molecular compounds, the concept of oxidation number was introduced.
Example: Hydrogen combining with oxygen to form water.
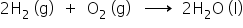
- In the formation of water, electron transfer is realised when hydrogen combines with oxygen.
- The hydrogen atom (H) goes from a neutral state, i.e. a zero state (H2) to a positive state in H2O, whereas the oxygen atom (O) goes from a neutral state, i.e. a zero state (O2) to a dinegative state in H2O.
- It is assumed that there is an electron transfer from H to O, and consequently, H2 is oxidised and O2 is reduced.
- The transfer of the charge is partial and considered an electron shift rather than a complete loss of electrons by hydrogen (H) and gain of electrons by oxygen (O).
Example:
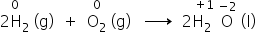
Answered by Ramandeep | 24 Sep, 2021, 17:21: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by Nehalakshmi65 | 14 Jul, 2024, 09:57: AM
CBSE 10 - Chemistry
Asked by shantilaljain03051973 | 09 Jul, 2024, 20:46: PM
CBSE 10 - Chemistry
Asked by ELECTRICAL.RCRC | 23 Jun, 2024, 04:23: AM
CBSE 10 - Chemistry
Asked by toppercontentteam | 18 Jun, 2024, 13:09: PM
CBSE 10 - Chemistry
Asked by psaisruthi10012009 | 07 Jun, 2024, 11:09: AM
CBSE 10 - Chemistry
Asked by pranathireddy736 | 28 May, 2024, 12:59: PM
CBSE 10 - Chemistry
Asked by karmvirsingh9602741719 | 17 May, 2024, 11:42: AM
CBSE 10 - Chemistry
Asked by kamalapallysudha17 | 25 Mar, 2024, 19:52: PM
CBSE 10 - Chemistry
Asked by sagarmishra | 04 Mar, 2024, 09:50: AM
CBSE 10 - Chemistry
Asked by 09.10bjanvhijadhav | 02 Mar, 2024, 08:22: AM