NEET Class neet Answered
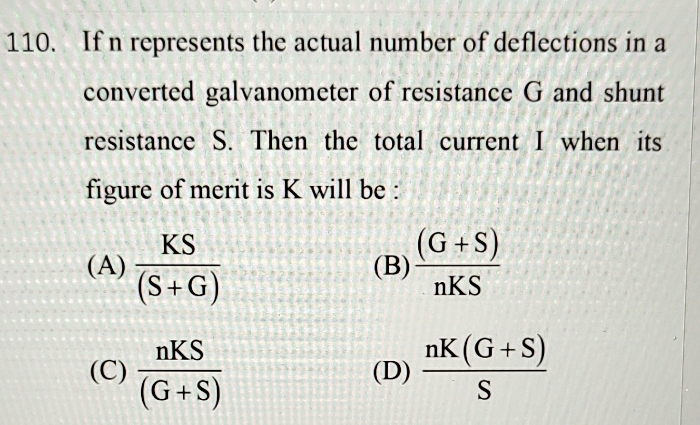
please answer this

Asked by Prashant DIGHE | 12 Jan, 2020, 09:20: PM
It is assumed that given power in the question is thermal power
Energy produced by reactor in one day = Power(W) × time(s) = 106 × 24 × 3600 J = 8.64 × 1010 J
Energy released per fission = 200 MeV = 200 × 106 × 1.602 × 10-19 J = 3.204 × 10-11 J
Number of fission reactions to produce 1 MW power in 1 day = ( 8.64 × 1010 / 3.204 × 10-11 ) ≈ 2.7 × 1021
Hence required number of 235U atoms = 2.7 × 1021
Required amount of 235U in mass = 2.7 × 1021 × [ 235 / ( 6.02 × 1023) ] = 1.05 g
Answered by Thiyagarajan K | 13 Jan, 2020, 10:16: AM
Application Videos
NEET neet - Physics
Asked by vk3460092 | 11 May, 2024, 05:31: PM
NEET neet - Physics
Asked by tejaskadiyan148 | 05 May, 2024, 11:52: AM
NEET neet - Physics
Asked by hardikmittal25 | 03 May, 2024, 02:57: PM
NEET neet - Physics
Asked by sa1033278 | 02 May, 2024, 07:37: PM
NEET neet - Physics
Asked by bidyutpravarout79 | 26 Apr, 2024, 09:40: PM
NEET neet - Physics
Asked by ramanjaneyuluoguru | 25 Apr, 2024, 04:18: PM
NEET neet - Physics
Asked by shatakshibhatt9 | 20 Apr, 2024, 07:52: PM
NEET neet - Physics
Asked by praveenpriya000079 | 18 Apr, 2024, 07:24: AM