NEET Class neet Answered
please answer this
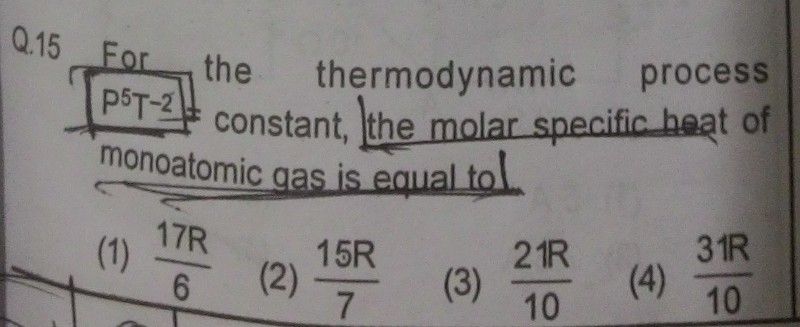
Asked by Prashant DIGHE | 01 Apr, 2020, 10:48: PM
Process Equation :- P5 / T2 = C , where C is constant, Hence P5 = CT2 ......................(1)
From first law of thermodynamics, we have, dQ = CvdT + P dV ......................(2)
For ideal gas of 1 mole, we have, P V = R T ......................(3)
By differentiating eqn.(3) , P dV + V dP = R dT .................(4)
We rewrite Eqn.(2) by Substituting PdV from eqn.(4)
dQ = CV dT + R dT - V dP .............................(5)
if we differentiate process eqn.(1), we get, 5 P4 dP = (2C) T dT
Hence from above eqn., we get, V dP = (2C / 5 ) P-4 V T dT = (2C/5) P-5 PV T dT ....................(6)
By substituting PV = RT and P5 = CT2 using eqn.(3) and (1), above eqn.(6) is simplified as,
V dP = (2/5) R dT ............................(7)
By substituting V dP using eqn.(7), we rewrite eqn.(5) as , dQ = CVdT + R dT - (2/5)R dT ....................(8)
for monoatomic gas, CV = (3/2)R
Hence Eqn.(8) becomes, dQ = [ (3/2)R + R - (2/5) R ] dT = (21/10)R dT
Hence molar specific heat = (21/10)R
Answered by Thiyagarajan K | 02 Apr, 2020, 07:18: AM
Application Videos
NEET neet - Physics
Asked by tejaskadiyan148 | 05 May, 2024, 11:52: AM
NEET neet - Physics
Asked by hardikmittal25 | 03 May, 2024, 02:57: PM
NEET neet - Physics
Asked by sa1033278 | 02 May, 2024, 07:37: PM
NEET neet - Physics
Asked by bidyutpravarout79 | 26 Apr, 2024, 09:40: PM
NEET neet - Physics
Asked by ramanjaneyuluoguru | 25 Apr, 2024, 04:18: PM
NEET neet - Physics
Asked by shatakshibhatt9 | 20 Apr, 2024, 07:52: PM
NEET neet - Physics
Asked by praveenpriya000079 | 18 Apr, 2024, 07:24: AM
NEET neet - Physics
Asked by gouranshi84 | 17 Apr, 2024, 05:23: PM
NEET neet - Physics
Asked by sojusvi | 17 Apr, 2024, 01:12: PM