NEET Class neet Answered

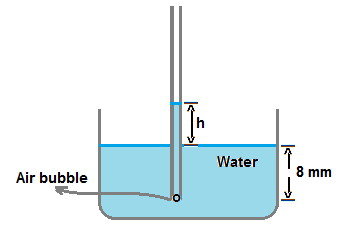
When the capillary tube of radius 2 mm is dipped inside water to a depth 8 mm , there is a capillary rise of water to a height h .
Height h of capillary rise is
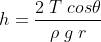
where T is surface tension of water , θ is angle of contact , ρ is density of water, g is acceleration due to gravity
and r is radius of capillary tube .
Angle θ of contact with water on glass is zero . Surface tension T of water is 0.072 N/m .
Hence height h of capillary rise is
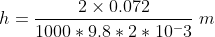
h = 7.3 × 10-3 m = 7.3 mm
If the bubble is at the end of capillary tube inside water as shown in figure,
then pressure inside the bubble is greater by (4T)/r than pressure outside bubble.
Pressure P inside the bubble is
P = Pa + [ ρ g (h+8) × 10-3 ] + [ (4T)/r ]
where Pa is atmospheric pressure .
If Pg is gauge pressure inside the bubble , then
Pg = P - Pa = [ ρ × g × (h+8) × 10-3 ] + [ (4T)/r ]
Pg = [ 1000 × 9.8 × (7.3 + 8.0 ) ] + [ ( 4 × 0.072 ) / 0.002 ] \ Pa
Pg = 294 Pa


