JEE Class main Answered
. Niobium crystallizes in bcc structure. If its density is 8.55 g/cm3. Calculate its edge length.
Atomic mass of Niobium = 93amu
Asked by remanikasohal24 | 14 Dec, 2020, 09:52: AM
Given:
No. of particles in b.c.c. type unit cell (Z) = 2
Atomic mass of the element (M) = 93 g mol−193 g mol-1
No. of particles in b.c.c. type unit cell (Z) = 2
Atomic mass of the element (M) = 93 g mol−193 g mol-1

Density of unit cell (ρ)=8.55g cm−3(ρ)=8.55g cm-3
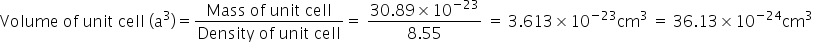
Edge length of unit cell (a) = (36.13×10−24cm3)1/3 = 3.31×10−8cm = 331 pm
Step II. Calculation of radius of unit cell

Answered by Ramandeep | 14 Dec, 2020, 12:37: PM
Concept Videos
JEE main - Chemistry
Asked by omgaikwad2412 | 25 Feb, 2022, 21:47: PM
JEE main - Chemistry
Asked by remanikasohal24 | 14 Dec, 2020, 09:52: AM
JEE main - Chemistry
Asked by ashutosharnold1998 | 16 Apr, 2020, 11:25: AM






