NEET Class neet Answered
Kindly explain (2) and (4).
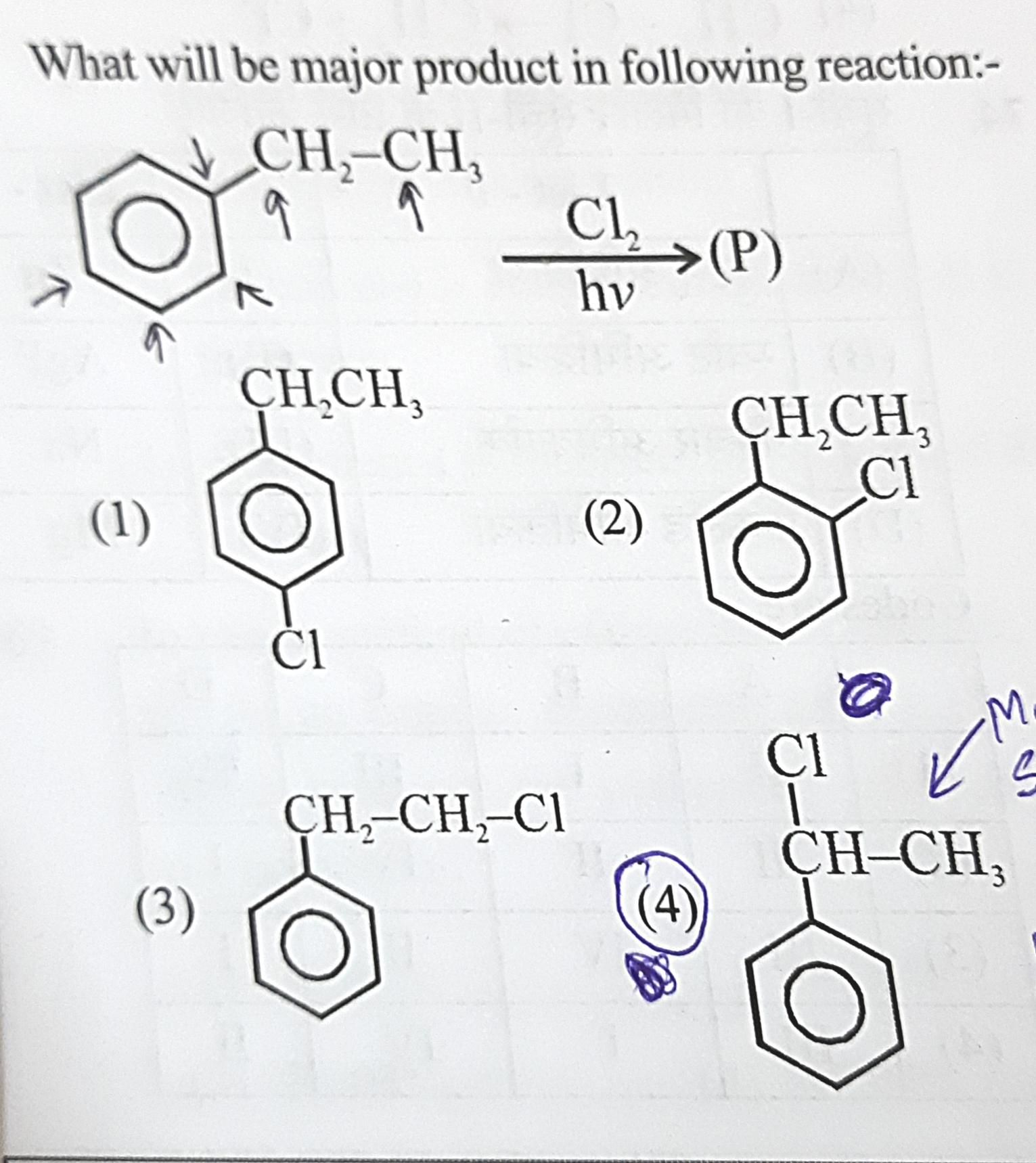
Asked by patra04011965 | 25 Jan, 2023, 11:09: AM
Dear Student,
1) Chlorination of ethylbenzene is a nucleophilic substitution reaction.
2) Formation of major product is determined by stability of carbocation formed.
3) When nucleophile attacks α C-atom, 2° carbocation is formed which is more stable. Hence, 1-chloro-1-phenylethane turns out to be major product.
4) When nucleophile attack β C-atom, 1° carbocation is formed which is less stable. Hence, 2-chloro-1-phenylethane turns out to be minor product.
2) Formation of major product is determined by stability of carbocation formed.
3) When nucleophile attacks α C-atom, 2° carbocation is formed which is more stable. Hence, 1-chloro-1-phenylethane turns out to be major product.
4) When nucleophile attack β C-atom, 1° carbocation is formed which is less stable. Hence, 2-chloro-1-phenylethane turns out to be minor product.
Answered by | 25 Jan, 2023, 02:41: PM
Application Videos
Concept Videos
NEET neet - Chemistry
Asked by biswassayan8464 | 21 Apr, 2024, 11:30: AM
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by raomayankup83 | 15 Apr, 2024, 07:46: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
Asked by vasantagomasi23 | 05 Apr, 2024, 08:35: AM
NEET neet - Chemistry
Asked by ankuruthanuriya | 03 Apr, 2024, 10:56: PM




















