CBSE Class 10 Answered
![]()
Step 1
In the first step, the ethanoic acid takes a proton (a hydrogen ion) from the concentrated sulphuric acid. The proton becomes attached to one of the lone pairs on the oxygen which is double-bonded to the carbon.
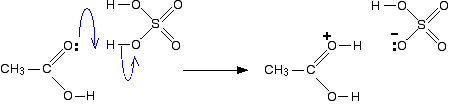
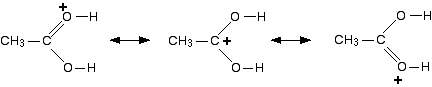
The transfer of the proton to the oxygen gives it a positive charge, but it is actually misleading to draw the structure in this way (although nearly everybody does!).
The positive charge is delocalised over the whole of the right-hand end of the ion, with a fair amount of positiveness on the carbon atom. In other words, you can think of an electron pair shifting to give this structure:
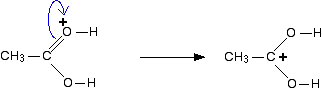
You could also imagine another electron pair shift producing a third structure:
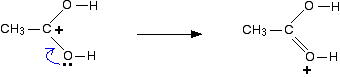
So which of these is the correct structure of the ion formed? None of them! The truth lies somewhere in between all of them. One way of writing the delocalised structure of the ion is like this:
Step 2
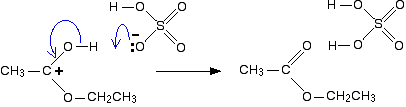
The positive charge on the carbon atom is attacked by one of the lone pairs on the oxygen of the ethanol molecule.
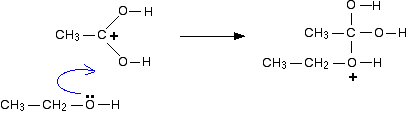
The net effect is:

Step 4
Now a molecule of water is lost from the ion.
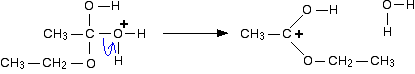
Step 5
The hydrogen is removed from the oxygen by reaction with the hydrogensulphate ion which was formed way back in the first step.