CBSE Class 9 Answered
First of all the need for mole. In very simple terms as you use litre and mL as a unit for measuring the volume, Kelvin or degree Celsius is taken as the unit for measuring temperature, similarly, for measuring the amount of the substance (element or compound) we use the term mole.
The idea of the mole to count entities at the microscopic level (i.e. atoms/molecules/ particles, electrons, ions, etc).
The definition of the mole is as:
One mole is the amount of a substance that contains as many particles or entities as there are atoms in exactly 12 g (or 0.012 kg) of the 12 C isotope.
When you are using the mole concept for solving numerical, we use it differently for an atom and a molecule.
For an atom,
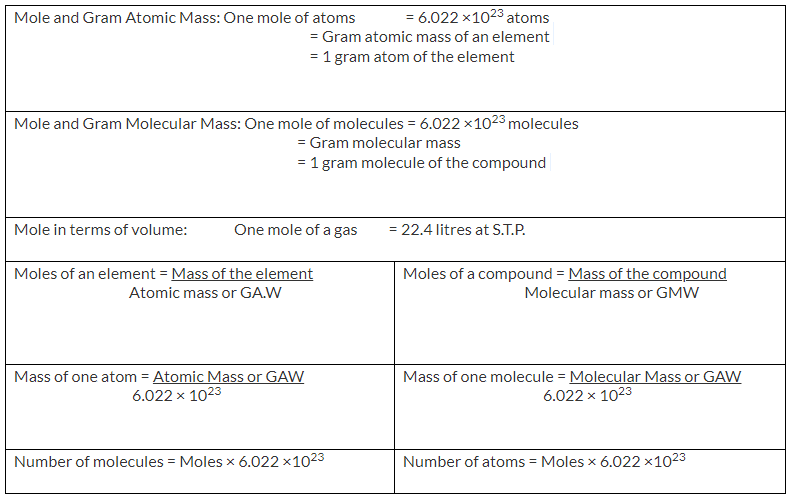
1 mole of an atom will contain 6.022 x 1023 atoms and the mass of 1 mole of an atom will be equal to its gram atomic mass.
So, we can write a simple equation as:
1 mole = 6.022 x 1023 atoms = Gram atomic mass
In a question, the relationship between the two quantities will be used. So, take the two quantities and leave the third one.
For example - if you are asked to calculate the mass of 2 moles of an oxygen atom, then take the relationship between moles and mass and apply the unitary method.
For a molecule,
1 mole of a molecule will contain 6.022 x 1023 molecules and the mass of 1 mole of the molecule will be equal to its gram molecular mass.
So, we can write a simple equation as:
1 mole = 6.022 x 1023 molecules = Gram molecular mass
In a question, the relationship between the two quantities will be used. So, take the two quantities and leave the third one. For example - if you are asked to calculate the number of molecules present in 20g of the oxygen molecule, then take the relationship between a number of molecules and mass and apply the unitary method.
Now there can be the third type of problems also in which you are asked to use the relationship like calculate the mass of sulphur atoms in 3 moles of Sulphur molecules. For such kind of questions, use the relationship as:
1 S8 molecules contain 8 S atoms
So, we can write the relationship as:
1 mole of S8 molecules = 8 moles of S atoms
Convert the number of moles to respective quantities you need
1 mole of S8 molecules = 8 x 32 g of S atoms
3 moles of S8 molecules = 8 x 32 x 3 g of S atoms
For stoichiometric numerical also use the balanced chemical equation and convert the equation into the relationship of moles as:
2H2 + O2 → 2H2O
We can write the relationship as:
2H2 + O2 → 2H2O
2 moles 1 mole 2 moles
Important formulae of mole concept:
This was the explanation of the mole concept. If you have any specific query on solving a particular numerical, do post a question.