CBSE Class 12-science Answered
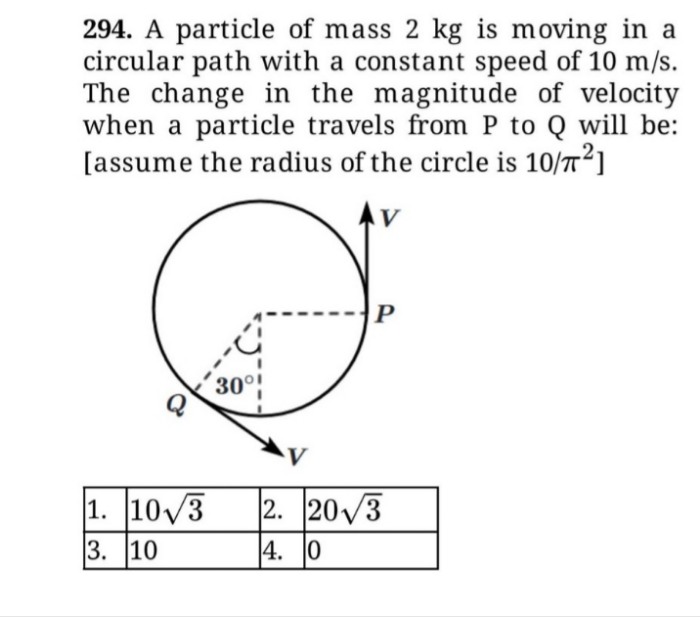
How much pressure be increased be increased in order to decrease the volume of a gas by 5% at a constant temperature?
Asked by Kushwahasms | 23 Sep, 2019, 04:48: AM
Pressure is inversely proportional to volume
Let Po and Vo be the initial pressure and initial volume.
let P and V be the final pressure and final volume .
( P / Po ) = ( Vo / V ) = 1/0.95
Hence P ≈ 1.053 Po ; Hence Pressure should be increased by 5.3%
Answered by Thiyagarajan K | 23 Sep, 2019, 09:43: AM
Concept Videos
CBSE 12-science - Physics
Asked by jothisugashini216 | 16 Jul, 2024, 20:30: PM
CBSE 12-science - Physics
Asked by prithviraj.chopra2011 | 14 Jul, 2024, 23:00: PM
CBSE 12-science - Physics
Asked by basithhhabduuu | 14 Jul, 2024, 17:07: PM
CBSE 12-science - Physics
Asked by asmaabid356 | 10 Jul, 2024, 21:03: PM
CBSE 12-science - Physics
Asked by pushpamagadum21 | 09 Jul, 2024, 20:43: PM
CBSE 12-science - Physics
Asked by sumitghorband09 | 20 Jun, 2024, 21:42: PM
CBSE 12-science - Physics
Asked by axonuploadserver4 | 14 Jun, 2024, 17:41: PM
CBSE 12-science - Physics
Asked by trishaktiprasadmallik693 | 11 Jun, 2024, 07:17: AM
CBSE 12-science - Physics
Asked by rahuldevgayen4 | 10 Jun, 2024, 14:58: PM