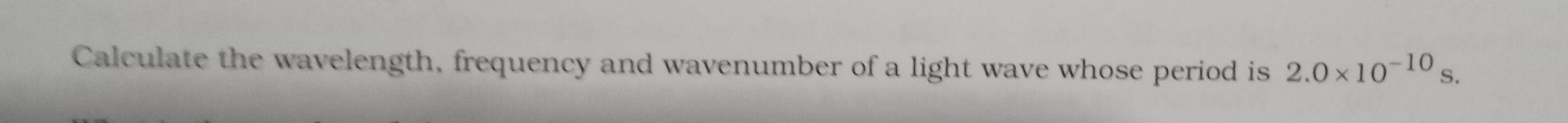JEE Class main Answered
Hi

Asked by jain.pradeep | 02 May, 2019, 23:53: PM
Electromagnetic radiation occurs from transition of electron from higher energy value (n+l) to lower energy value.
Option (E) is correct.
Transition III
3d(n+l=5) → 3p(n+l=4)
Transition V
4s(n+l=4) → 2p(n+l=3)
These two radiations results in the emission of electromagnetic radiatios.
Answered by Varsha | 03 May, 2019, 11:36: AM
JEE main - Chemistry
Asked by radham6375 | 17 May, 2024, 20:13: PM
JEE main - Chemistry
Asked by 9079344910choudhary | 16 May, 2024, 18:52: PM
JEE main - Chemistry
Asked by purnendurai26 | 02 May, 2024, 18:34: PM
JEE main - Chemistry
Asked by cheekatiyogendra143 | 20 Apr, 2024, 11:16: AM
JEE main - Chemistry
Asked by jwhhebbb | 19 Apr, 2024, 13:21: PM
JEE main - Chemistry
Asked by adityadoodi3 | 05 Apr, 2024, 23:27: PM
JEE main - Chemistry
Asked by pratap62437 | 19 Feb, 2024, 12:48: PM
JEE main - Chemistry
Asked by sayushman087 | 01 Feb, 2024, 10:28: AM
JEE main - Chemistry
Asked by marthalamanoharreddy65 | 17 Dec, 2023, 10:26: AM