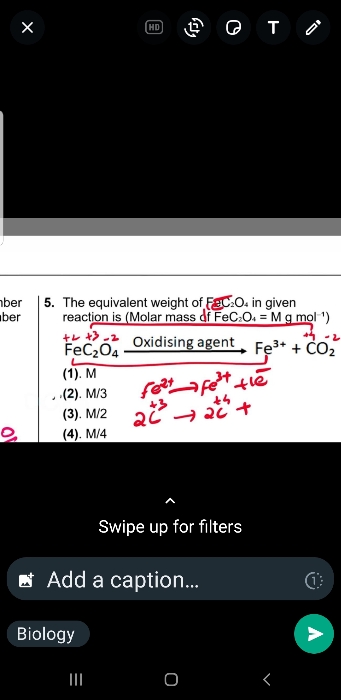
NEET Class neet Answered
find the persenties o,c,h in one mole of glucos
Asked by rohithindustani56922 | 10 Jul, 2019, 06:47: PM
Molar mass of glucose (C6H12O6) = 180 g/mol
Atomic mass of O = 16
Atomic mass of H = 1
Atomic mass of C = 12
1 mole of glucose = Molar mass of glucose = 180 g
So, 180 gm of glucose contains 96 gm of oxygen
Therefore 100 gm of glucose will contain,
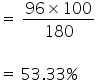
180 gm of glucose contains 6 gm of hydrogen
So % of hydrogen is
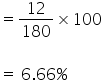
180 gm of glucose contains 72 gm of carbon
So % of carbon is
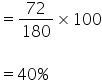
Percentage composition of C, H and O in glucose is 40%, 6.66% and 53.33% respectively.
Answered by Varsha | 11 Jul, 2019, 12:13: PM
NEET neet - Chemistry
Asked by sahumansi | 12 May, 2024, 09:37: AM
NEET neet - Chemistry
Asked by biswassayan8464 | 21 Apr, 2024, 11:30: AM
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
Asked by vaka.aruna1979 | 23 Mar, 2024, 04:18: AM
NEET neet - Chemistry
Asked by fathimahusna23042004 | 03 Mar, 2024, 08:56: AM
NEET neet - Chemistry
Asked by drkeshavkhandagle | 18 Jan, 2024, 08:10: PM
NEET neet - Chemistry
Asked by yogitakumawat | 21 Dec, 2023, 10:31: PM