NEET Class neet Answered
Find the most steam volatile species.
(Please see attached question and explain in details)
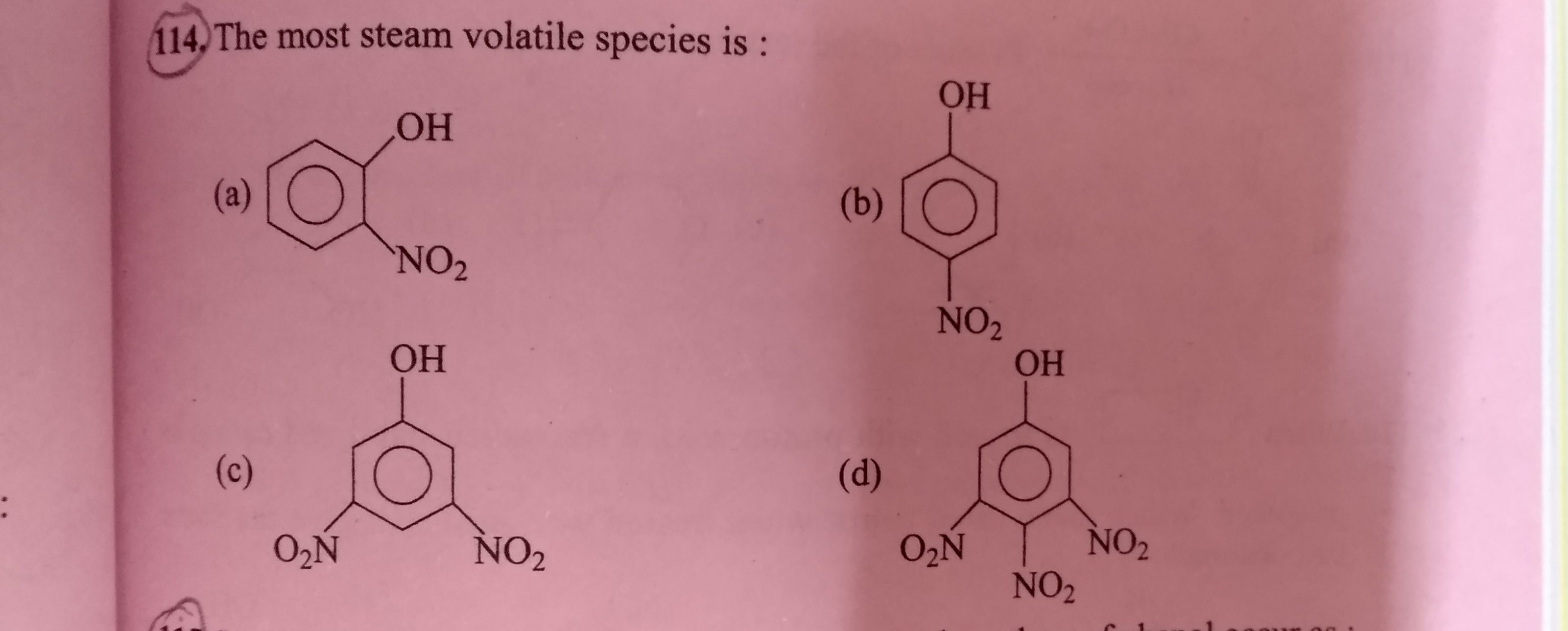
Asked by subhrojyotighosh8 | 20 Jul, 2020, 10:44: PM
Melting and boiling points of any solid or liquid are depend on the hydrogen bonding between the molecules.
In this case o-nitrophenol and p-nitrophenol both shows hydrogen bonding.
hydrogen bonding has two types intermolecular(between two molecules) and intramolecular(within the molecule) hydrogen bonding.
Intermolecular bonding is stronger hence compounds having intermolecular bonding have higher boiling points, whereas its mostly opposite in intramolecular bonding.
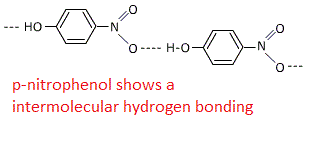
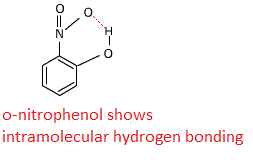
Due to inramolecular bonding in o-nitrophenol is more steam volatile than p-nitrophenol.
Answered by Ramandeep | 21 Jul, 2020, 12:16: PM
NEET neet - Chemistry
Asked by dipakmodi2309 | 17 Dec, 2022, 02:00: AM
NEET neet - Chemistry
Asked by subhrojyotighosh8 | 20 Jul, 2020, 10:44: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 19 Feb, 2020, 10:44: PM
NEET neet - Chemistry
Asked by Karthika.m.nair01 | 05 Mar, 2019, 01:18: PM


