NEET Class neet Answered
Explain.
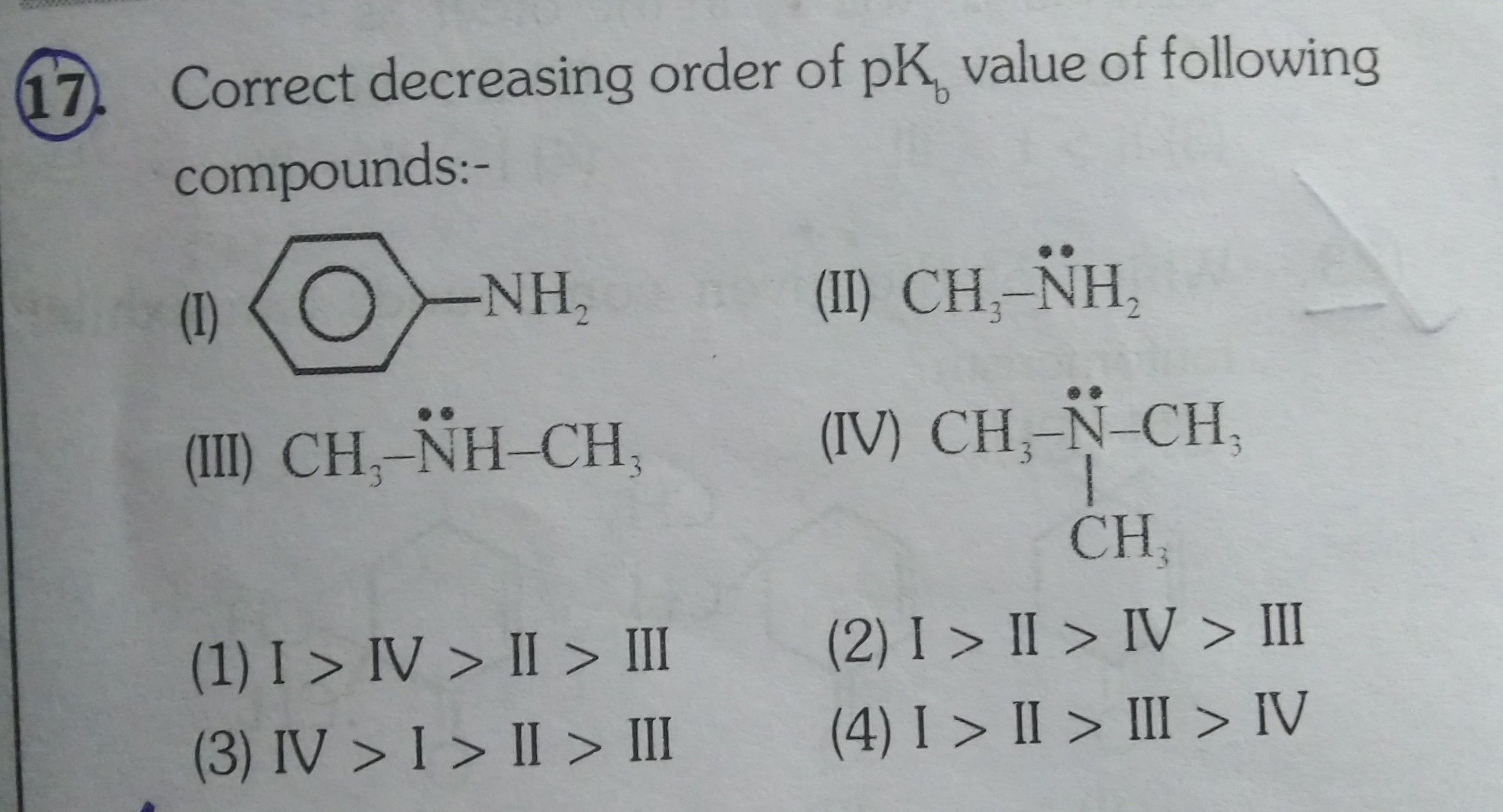
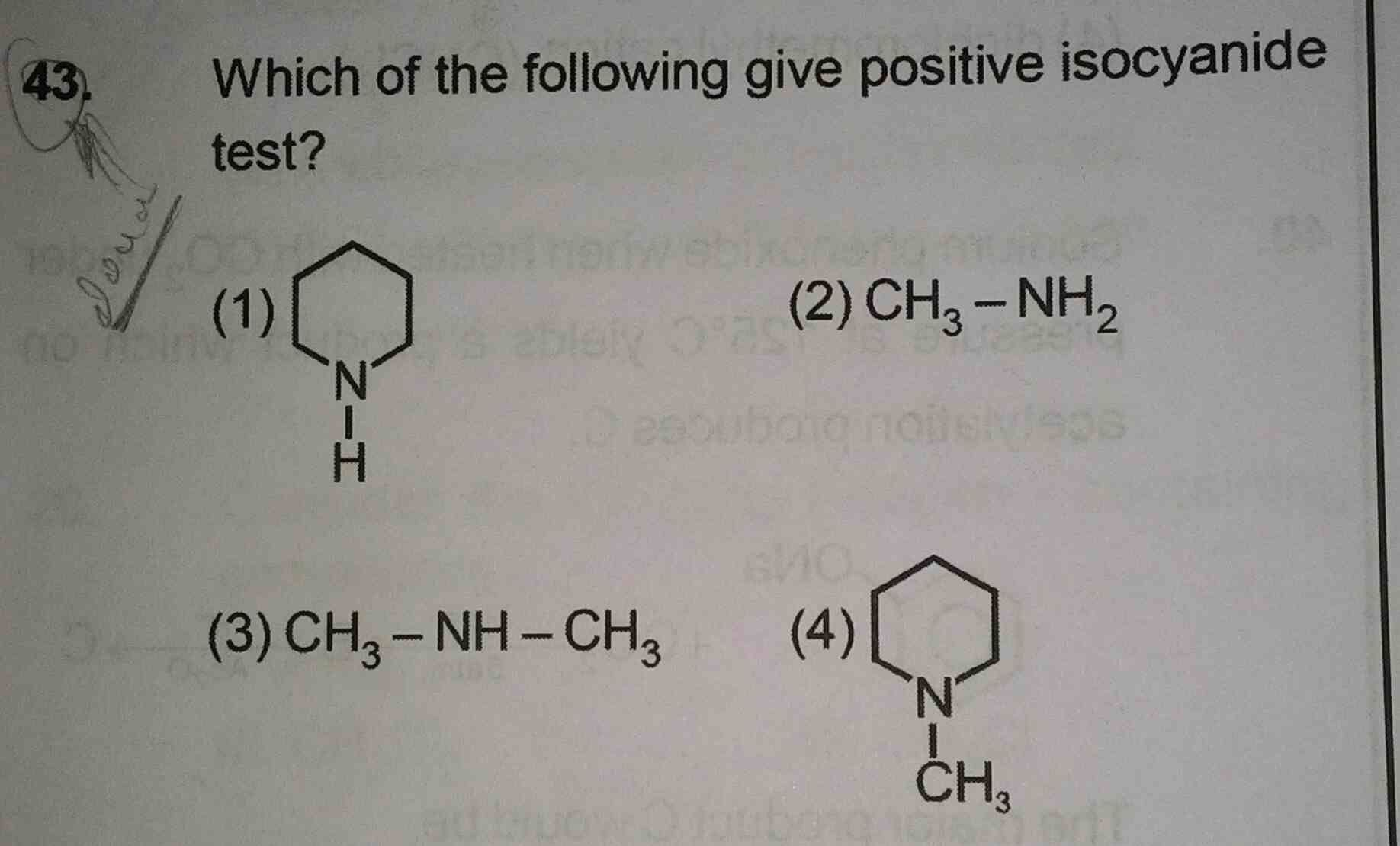
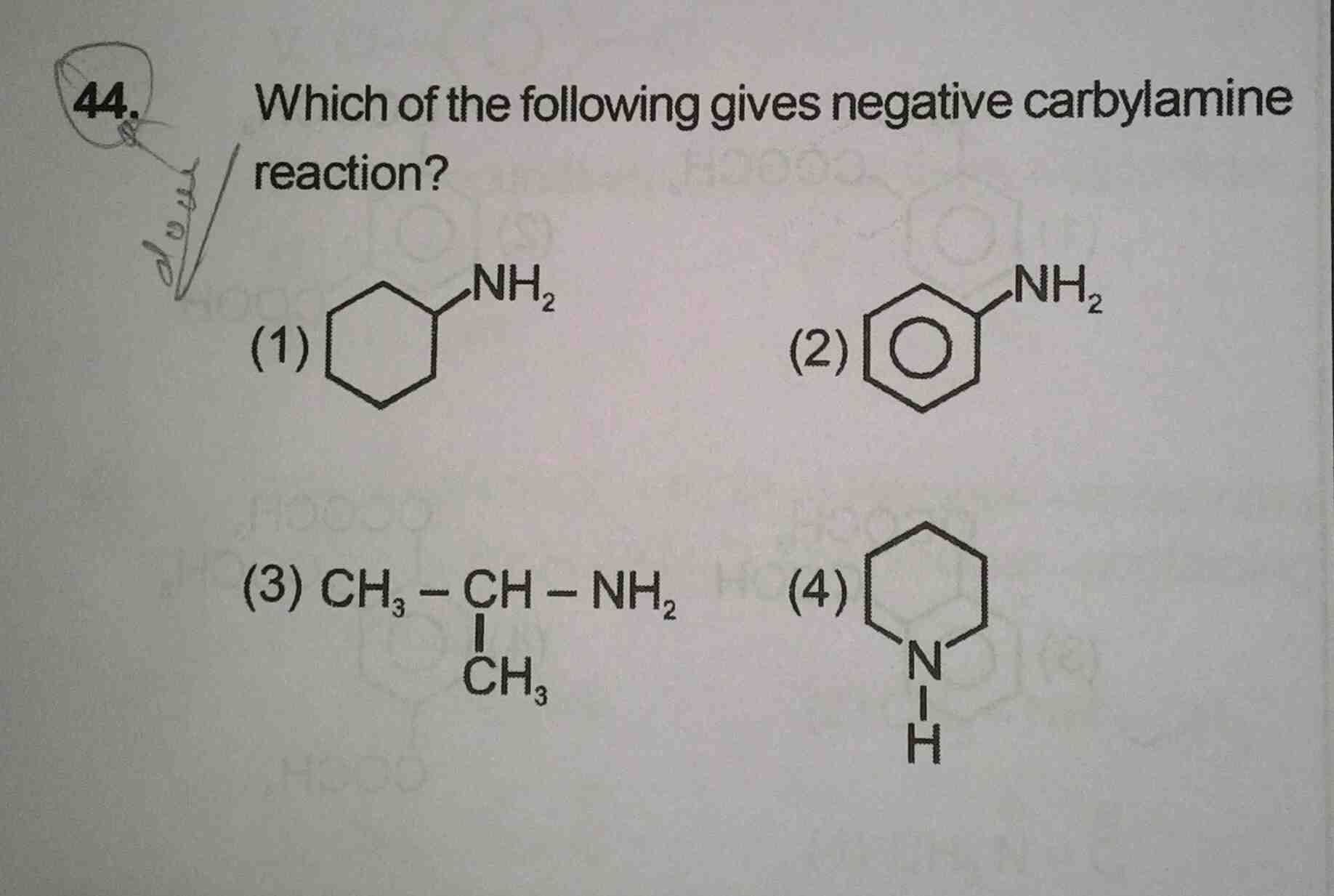
Asked by musira29rahman | 03 Sep, 2019, 04:48: PM
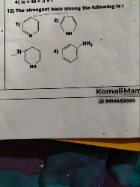
A base is said to be strong base (tendency to donate electrons) has smaller pkb value.
As we know that aliphatic amines (R-NH2) are more basic as compared to aromatic amine (Ph-NH2 less availability of electrons due to resonance).
More the alkyl group are present on the nitrogen more the electron density increases on nitrogen.
Hence the correct decreasing order of pkb value of given compounds is:
C6H5NH2 > CH3NH2 > CH3NHCH3 > (CH3)3N
Answered by Ramandeep | 03 Sep, 2019, 06:15: PM
NEET neet - Chemistry
Asked by ankuruthanuriya | 03 Apr, 2024, 10:56: PM
NEET neet - Chemistry
Asked by murtazan92 | 20 Nov, 2023, 11:45: PM
NEET neet - Chemistry
Asked by nagendrakakumuri | 19 Nov, 2023, 10:53: AM
NEET neet - Chemistry
Asked by vanshkamboj598 | 17 Aug, 2022, 02:37: AM
NEET neet - Chemistry
Asked by rohitraman1115 | 16 Feb, 2021, 08:16: PM
NEET neet - Chemistry
Asked by nameerasiddiquee90 | 05 Feb, 2021, 04:19: PM
NEET neet - Chemistry
Asked by subhrojyotighosh8 | 17 Jul, 2020, 11:12: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 27 Feb, 2020, 09:50: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 27 Feb, 2020, 09:44: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 26 Feb, 2020, 10:04: PM