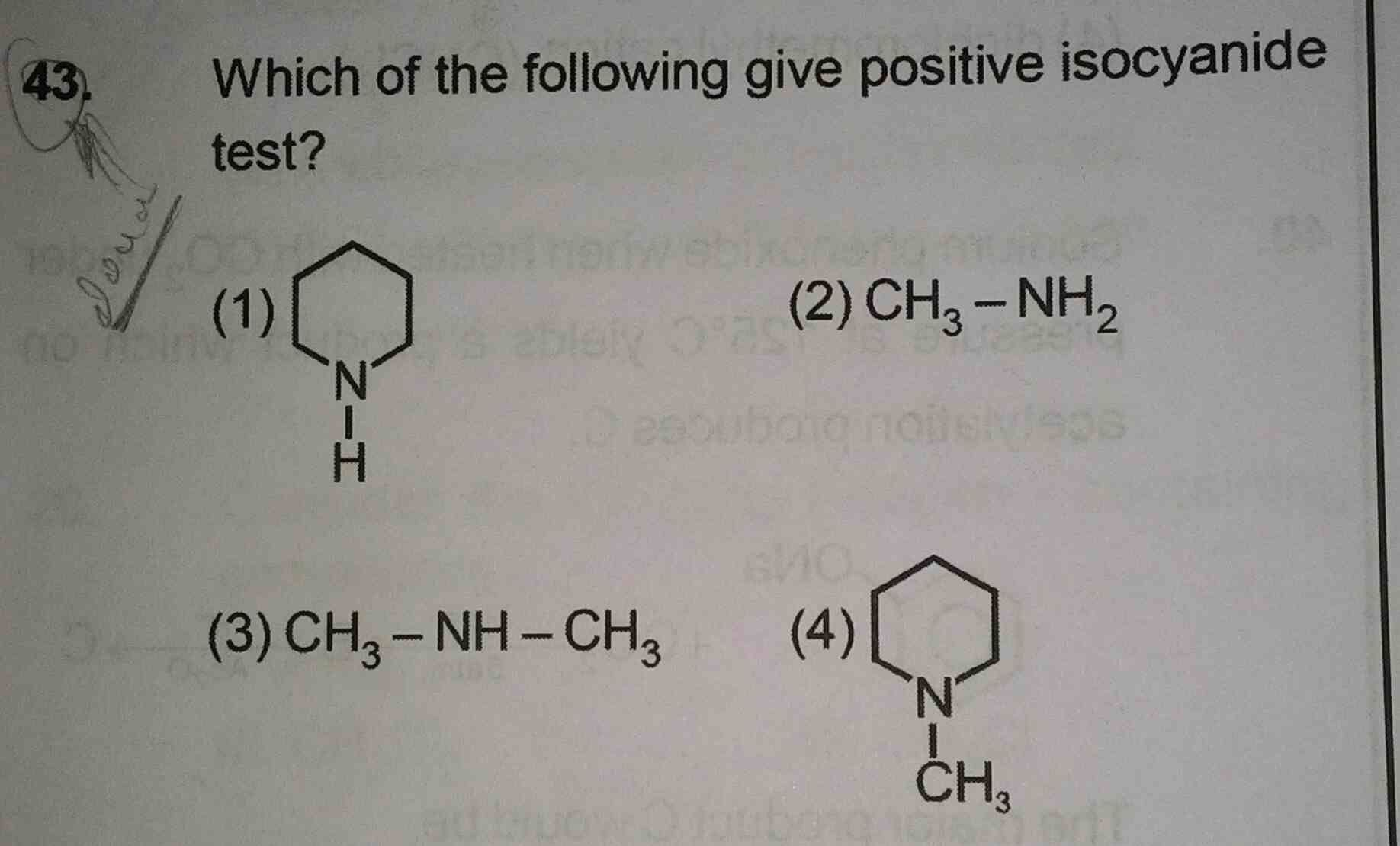
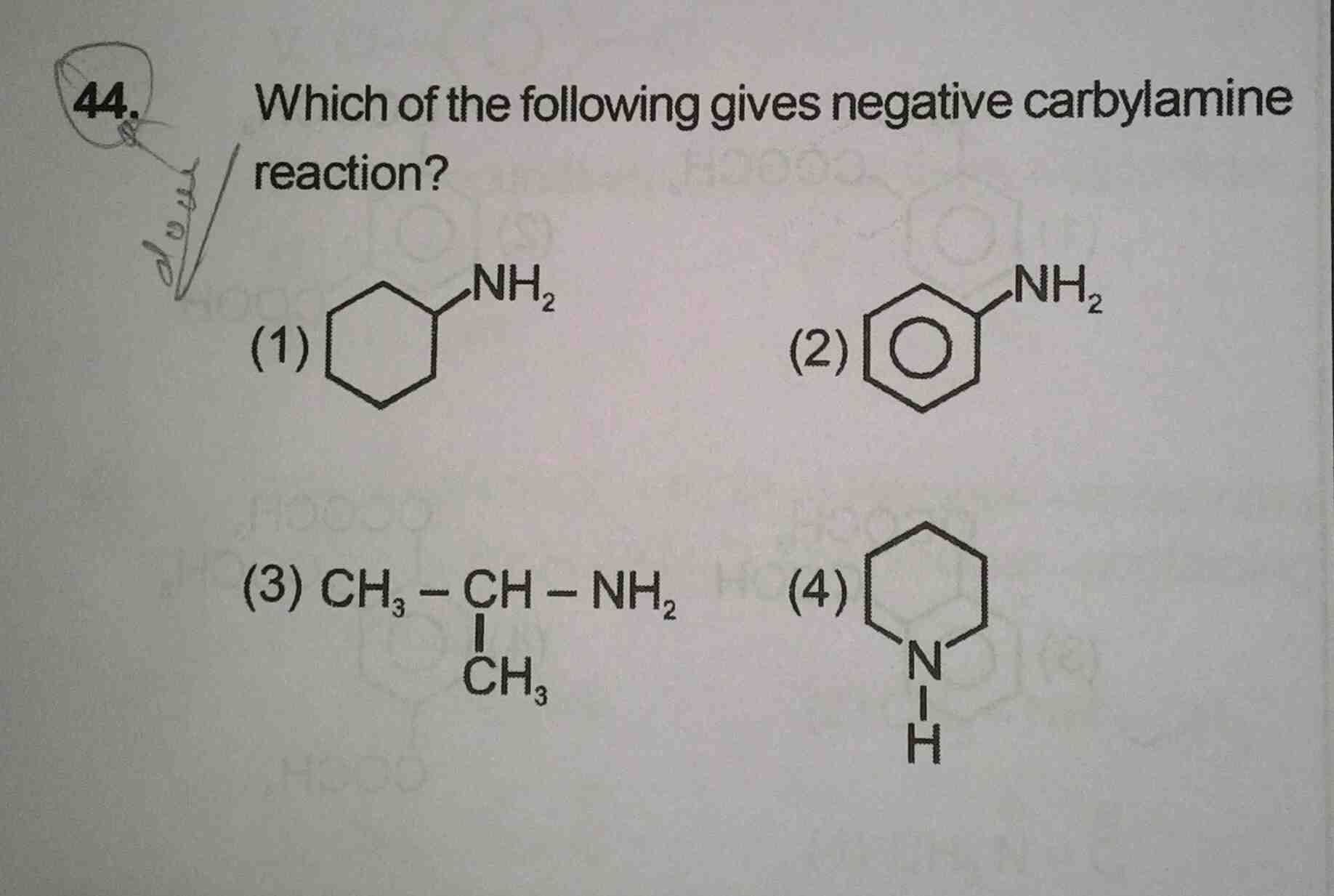
NEET Class neet Answered
35 se all questions ke ans plzz now

Asked by murtazan92 | 20 Nov, 2023, 23:45: PM
Dear Student,
Answer for query no. 35
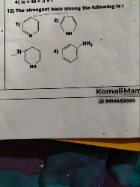
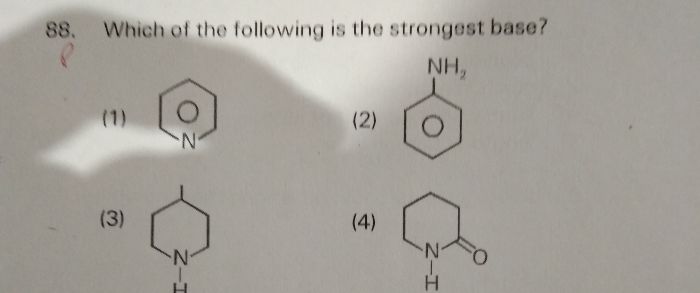
The order of basicity of given compounds is
(A) C6H5NH2 < (B) CH3NH2 < (D) (CH3)3N < (C) (CH3)2NH
Methyl amine is more basic than Aniline since lone pair of electrons on nitrogen in aniline is attracted towards stable aromatic nucleus so less available.
More the electron donating alkyl groups, more the basicity, so methylamine is less basic than di and tri methyl amines.
Dimethyl amine is more basic than trimethylamine because of steric crowding of methyl groups in trimethylamine.
Kindly ask one query at a time so that we can help you to clear your doubt.
Answered by | 21 Nov, 2023, 09:49: AM
NEET neet - Chemistry
Asked by ankuruthanuriya | 03 Apr, 2024, 22:56: PM
NEET neet - Chemistry
Asked by murtazan92 | 20 Nov, 2023, 23:45: PM
NEET neet - Chemistry
Asked by nagendrakakumuri | 19 Nov, 2023, 10:53: AM
NEET neet - Chemistry
Asked by vanshkamboj598 | 17 Aug, 2022, 02:37: AM
NEET neet - Chemistry
Asked by rohitraman1115 | 16 Feb, 2021, 20:16: PM
NEET neet - Chemistry
Asked by nameerasiddiquee90 | 05 Feb, 2021, 16:19: PM
NEET neet - Chemistry
Asked by subhrojyotighosh8 | 17 Jul, 2020, 23:12: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 27 Feb, 2020, 21:50: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 27 Feb, 2020, 21:44: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 26 Feb, 2020, 22:04: PM