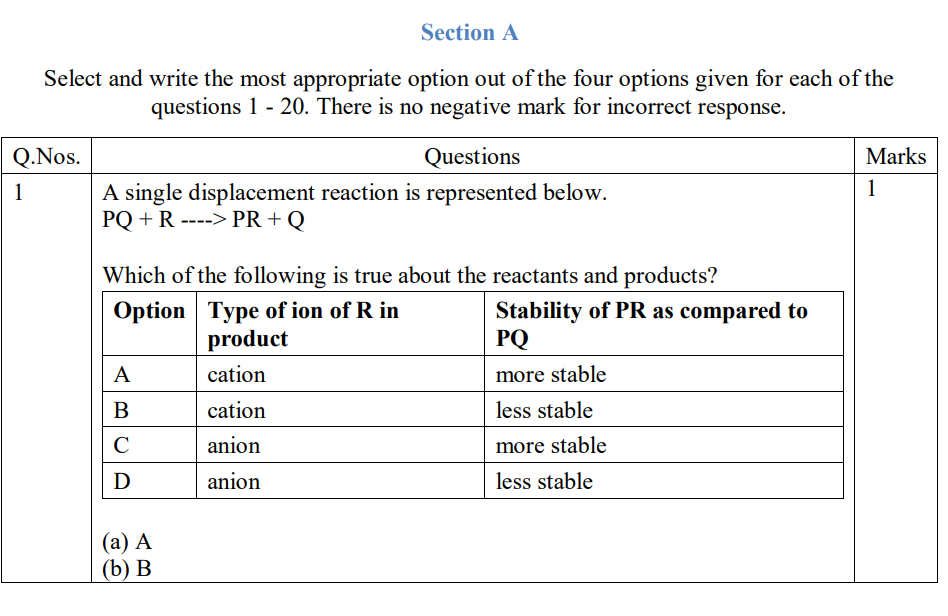
CBSE Class 10 Answered
Displacement reaction:The reaction in which one element displaces another element from its compound is known as displacement reaction. In these reactions, more reactive element displaces less reactive element from its compound.
Example- 2AgNO3 (aq) +Cu Cu (NO3)2 (aq) + 2Ag (S)
Silver is replaced by copper because copper is more reactive than silver.
Double displacement reaction: The reaction in which two different atoms or group of atoms (ions) are displaced by other atoms or group of atoms (ions) is known as double displacement reaction.
Example Na2SO4(aq) + BaCl2 (aq) BaSO4(s) + 2NaCl(aq)
Here, SO42- displaces Cl- ions and Cl- ions displace SO42- ions or both ions exchange each other. These reactions occur in ionic compounds.